What AnteAge Doesn’t Know and Therefore Doesn’t Tell You
Consider the stem cells used by AnteAge to make their products: Bone Marrow Mesenchymal Stem Cells (BMSCs) and the molecules they release prolong and enhance inflammation by increasing survival and function of neutrophils (Castella et al, 2011). Under hypoxic conditions, which induces the activation of TRL4, BMSCs secrete pro-inflammatory factors and decrease the polarization of macrophages from the M1 to M2 phenotype (Faulknor et al, 2017; Waterman et al, 2010). Therefore, BMSCs cultured in normal hypoxic conditions in the laboratory are secreting pro-inflammatory factors and when administered to wounded skin will induce inflammation by recruiting neutrophils and M1 type pro-inflammatory macrophages. When you put AnteAge on your skin, these are the pro-inflammatory molecules damging your skin. In contradistinction, consider the cells used by Neogenesis. The phenotype and stem cell released molecules (also called the secretome) from skin-derived adipose mesenchymal stem cells (AMSCs) are largely unaffected by prolonged hypoxia, not recruiting neutrophils (Kalinina et al, 2015), and the molecules released from AMSCs were found to better induce the effects of the anti-inflammatory M2 macrophage phenotype than the molecules released from BMSCs (Sukho et al, 2018). These results provide strong evidence that the molecules released from AMSC are more beneficial than those from BMSCs to induce appropriate wound healing processes through the shift from a pro-inflammatory state to an anti-inflammatory, pro-healing state.
These pro-inflammatory signals from BMSC cytokines are in addition to their likelihood of containing pro-oncogenic signals that are absent in AMSCs, that I have previously reviewed in multiple, peer-reviewed, National Library of Medicine journal articles (Maguire, 2019; 2021; 2022).
Why does AnteAge use these inflammatory and potentially oncogenic molecules in their products? The first clue comes from who founded the company and was its CEO for years, John Sanderson. A former physician who lost his medical license because of incompetence, repeated incompetence, and sexual misconduct with one of his patients.

Sanderson previously was a family practice physician with an undergraduate medical degree – a bachelor’s degree in medicine. Once Sanderson finished his Canadian undergraduate degree in medicine, once he passed the medical board test in the US, regulations permitted him to use the designation “M.D.” Sanderson frequently finds himself in disputes with other companies, one of which exposed that Sanderson committed domestic violence. He was not trained as a dermatologist and was not board certified. He obviously has little to no understanding of the skin’s powerful immune system, and no idea of how bone marrow mesenchymal stem cells work in the body. Upon losing his medical license, he started a company to do further harm to people by having them use products that induce inflammation and potentially cancer.
Trying to understand why a company would bring a proinflammatory, possibly pro-oncogenic product to the market, I looked closer at the company. Because John Sanderson is not a scientist, and has never listed that he has any scientific publication, only misleading blogs, I wondered how did he come to choose his technology. I discovered that Sanderson had enlisted fellow Canadian, Jonathan Lakey, Ph.D. as his scientific advisor. To no surprise, the man who had lost his medical license because of incompetence had hired a scientist, Jonathan Lakey, who had been fired from his university because of fraud.

A non-profit government organization in Canada fired Jonathan Lakey for the same reason:
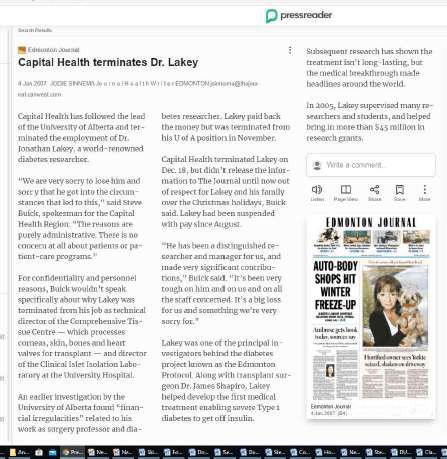
Then Jonathan Lakey was charged with fraud and racketeering at one of the companies in which he was an officer:

Jonathan Lakey’s involvement with a number of other companies that are pump and dump schemes has made the news a number of times. Clearly, using a product on your skin from this dynamic fraudster-incompetence duo is a bad choice – they do not have anyone’s well being in mind.
There are other skin care companies led by physicians. I suggest if you’re interested in their products, go to the state medical board website in which they practice, and look at the current status of their medical license. For example, you can search physicians in California here, and in Colorado here. You may be surprised what you find. Simply type in their name, and you’re likely to find disiplinery actions and loss of license.
References
Castella M.A. et al (2011) Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 29:1001–1011.
Faulknor R.A. et al (2017) Hypoxia impairs mesenchymal stromal cell-induced macrophage M1 to M2. Technology. 2017;5:81–86. doi: 10.1142/S2339547817500042.
Kalinina N. et al. (2015) Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. Ther. ;6:221.
Maguire G. Transplanted stem cells survive a long time: do they make you sick? J R Soc Med. 2019 Oct;112(10):412-414.
Maguire G. (2021) Stem cells part of the innate and adaptive immune systems as a therapeutic for Covid-19. Commun Integr Biol. 14(1):186-198.
Maguire G. (2022) Chronic inflammation induced by microneedling and the use of bone marrow stem cell cytokines. J Tissue Viability. 31(4):687-692.
Sukho P et al (2018) Human mesenchymal stromal cell sheets induce macrophages predominantly to an anti-inflammatory phenotype. Stem Cells Dev. 27:922–934.
Waterman R.S. et al (2010) A new mesenchymal stem cell (MSC) paradigm, polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE. 5:e10088.
3 thoughts on “Why Skin-Derived, Adipose Mesenchymal Stem Cell Released Molecules (NeoGenesis) Are Better Than Bone Marrow Mesenchymal Stem Cell Cytokines (AnteAge) for Post-Procedure Healing”