Please stop using D- and L- because it shows your profound ignorance. And, yes, both R- and S-Mandelic Acid work in the body. And, please don’t use mandelic acid with only one enantiomer as some companies want you to – it’s toxic! And please don’t drink your sunscreen – it’s stupid!
Organic compounds, molecules created around a chain of carbon atom, commonly known as carbon backbone, play an essential role in the chemistry of life. The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with other elements but also with itself. Because of its position midway in the second horizontal row of the periodic table, carbon is neither an electropositive nor an electronegative element; it therefore is more likely to share electrons than to gain or lose them. In other words, it’s stable and doesn’t require the gain or loss of electrons, yet permissive because it has 4 electrons to share. Of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Other elements, such as phosphorus [P] and cobalt [Co], are able to form five and six covalent bonds, respectively, with other elements, but they lack carbon’s ability to bond indefinitely with itself. As such, carbon can form an extensive number of molecules types, and indeed, very complex molecules. These molecules are important in the energy they carry, mainly in a form of potential energy between atomic molecules. Since such potential force can be widely affected due to changes in atomic placement, it is important to understand the concept of an isomer, a molecule sharing same atomic constituents as another but differing in structural arrangement.
Stereoisomers are isomers that differ in spatial arrangement of atoms, rather than order of atomic connectivity. One type of isomer is the mirror-image stereoisomers, a non-superimposable set of two molecules that are mirror image of one another. The existence of these molecules are characterized by a concept known as chirality.
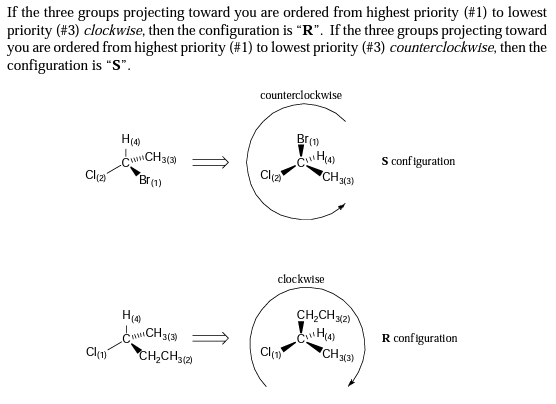
D and L configuration is an old classification that is rarely used by scientists, but is commonly used by dilletantes in the popular press and cosmetic companies who are clueless about chemistry, especially stereochemistry. D- and L- as incorrectly used by dilletantes is said to have been used to point out optical rotation in the early days that preceded a knowledge of stereochemistry. Unbeknownst to the dilletantes is that optical rotation is indicated by lowercase “d” and “l”, not uppercase. The modern way of describing chirality of molecules is the R-S system. If you want to understand how chirality is determined using the R,S system, here’s a simple guide, and a summary diagram is given below.
How did D and L originate, and why the confusion? D and L were originally used by Prof. Dr. Emil Fischer, Ph.D., in about 1891, when he was busy determining the configurations of all the sugars (glucose) by building them from d-glyceraldehyde, which has one chiral center. Chiral centers were already known to exist, although scientists were not able to determine their absolute configuration until about 1951. The idea of the chiral center was developed by a Dutch chemist, Prof. Dr. Jacobus Henricus van ‘t Hoff, Ph.D., in 1874, the first scientist to be awarded the Nobel prize in chemistry.
Prof. Dr. Emil Fischer, Ph.D., working in Germany, decided that d-glyceraldehyde was what we would now call “R”. Since his method of building sugars from D-glyceraldehyde preserved the original chiral center, he was able to determine that all the sugars he built also had D configuration in their last chiral center; that’s why we talk of “D-sugars.” In other words, building from a D-sugar led to the building of other D-sugars. Big D and Little D simply referred to sugars built from d-glyceraldehyde.
Back in the day when I lectured students about biochemistry, I would introduce D and L as a historical fact that they will have to deal with because of misinformation by non-chemists in the medical literature. Let’s review:
We now use a different system that is absolute in determining the chirality of molecules, and it uses R and S. Here’s a summary:
While the chiral (R)-mandelic acid (R-MA) is important for the drug industry because it’s a useful chiral building block for the synthesis of aromatic drugs, it is of crucial importance in the chemical and pharmaceutical industry. In humans, S-mandelic acid undergoes rapid chiral inversion to R-mandelic acid. So a racemic mixture of the R- and S- enantiomers is of little importance when using mandelic acid for topical skin applications, even if the R- and S- were shown to have different effects – and they haven’t been found to have different effects. Chiral chemicals often have functionality in both enantiomers, often with the function of each enantiomer acting at different pathways. The function of R- versus S- for many chemicals, including drugs, has not been determined.
Possible Toxicity of Using Mandelic Acid With Only One Steroisomer (enantiomer)
Here’s why you don’t want to use pure R-mandelic acid. (R)–MA is mainly synthesized chemically. The cyanide-based method involved two-step reactions including cyanation of benzaldehyde using either NaCN or transition metal catalysts, such as titanium or vanadium complexes of chiral ligands, followed by the hydrolysis of mandelonitrile using HCl to give enantiopure (R)–MA (Blacker and Houson 2002; Corson et al. 2003). This method requires the use of highly toxic cyanide and expensive transition metal catalyst together with chiral ligands but gives unsatisfied ee (meaning it doesn’t yield enantiomer excess, i.e. it doesn’t yield just the R- enantiomer), low overall yields, and generates a lot of by-products and large amount of waste. The dichloroacetophenone-based method involved the chlorination of acetophenone with chlorine, followed by alkaline hydrolysis by NaOH at 65 °C and the acidolysis with HCl. This method requires the use of toxic and dangerous Cl2, high temperature, and also suffers from side products problems (mono, tri-choloroacetophenone) (Aston et al. 2003).
Bottom line, and practically speaking, chemical methods are mainly used to produce racemic Mandelic Acid (not chiral Mandelic Acid) because it works, it’s clean (not toxic), it’s inexpensive, and it’s sustainable. It makes sense.