Failed company brings suboptimal skincare product to market by a manufacturer in Korea, known for fraudulent and corrupt stem cell science, even at it’s premier university, Seoul National University.
Benev is a company that couldn’t survive in the market, and had numerous problems as exemplified by this FDA review where they found poor quality control and the use of expired materials being used in production:
http://fda-warning-letters.blogspot.com/2010/06/benev-company-inc.html
For example, Benev was using expired ingredients to manufacture drugs that went to market, and falsified documents to hide their egregious behavior (below is from a FDA Warning Letter to Benev):

“QCD” refers to Benev’s quality control department.
As a consequence of this violation, and may other violations, FDA concluded that Benev’s drug products were adulterated:
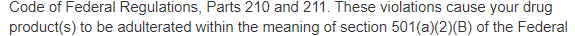
Resulting from continued poor performance, Benev sold themselves to a Korean company, ExoCoBio, that uses Benev to sell exosomes in the USA. A culture of corruption and fraud was highlighted by the veterinarian, Hwang Woo-suk, and his many conspirators who faked a landmark stem cell publication. That culture was exported to the USA by Benev. Previously, Benev worked with another local company called Invitrx, a company with a rich history of FDA violations, a long history, and led by a man, Habib Torfi, known for delivering stem cells to patients in a grocery bag.
There are numerous problems with what Benev (ExoCoBio) is doing to exosomes.
First, exosomes are only a fraction of what stem cells release, and without the non-exosomal fraction being combined with the exosomes, suboptimal results are achieved. In other words, when the exosomes aren’t isolated but are combined with the soluble fraction as is natural when the stem cells release their molecules, the results are superior to using only the isolated exosomes.
Second, they lyophilize their exosomes – this is a freeze-drying process that damages the molecules inside the exosome, and molecules attached to the outside of the exosome. Basically this harsh process removes all the water from the product, leaving a small amount of dry powder. The powder is full of damaged proteins and other molecules. Lyophilization leads to aggregation of proteins and their denaturization. “Unfortunately, the lyophilization process generates both freezing and drying stresses, which can denature proteins to various degrees” (Wang, 2000). Protein denaturation refers to the loss of biological activity through changes of the specific spatial conformation of protein in certain physical or chemical factors, resulting in the change of physical and chemical properties.
Lyophilization is used for the convenience of the company – it’s easier to store and ship a small pellet of lyophilized powder than it is to store and ship fresh, undamaged exosomes contained in their original solution.
From the Benev website we see they’re using lyophilized exosomes, and only the exosomes without the benefit of the soluble fraction (the overlapping text on their website is another example of the lack of attention to detail in this failed company):

To compare, NeoGenesis uses fresh (not damaged from freeze-drying) S2RM that contains both the 1. exosomal fraction, and 2. soluble fraction. Also, NeoGenesis uses both fractions from 3 cell types derived from the skin (mesenchymal stem cells and two types of fibroblasts). In contrast, Benev uses only a portion of the molecules released from one cell type, yielding a much depleted set of molecule types compared to NeoGenesis, many of which are damaged by Benev using lyophilization.
Another nearby company, Invitrx, in Lake Forest, is selling non-sterile exosomes for injection – allogenic injection. Talk about dangerous. Invitrx has a long history of unsafe practices. For example, illegally selling stem cells for injection, delivered in a paper grocery bag and selling non-sterile exosomes to physicians as exemplified in this FDA 486 Warning Letter, in which the agency details numerous non-sterile practices used to produce their exosomes.
You can read about the problems with lyophilization with many references to the published literature in my blog:
One thought on “Suboptimal Exosome Product Brought to Market By Failed Company – Benev, and Another Local Company – Invitrx”