A new study analyzing 23 randomized clinical trials with 1474 participants has found that “collagen supplements” significantly improved skin hydration, elasticity, and wrinkles only if you include data from physician-investigators who were paid by pharmaceutical/supplement companies. However, an analysis of studies not receiving funding from pharmaceutical companies revealed no effect of collagen supplements for improving skin hydration, elasticity, and wrinkles, while those receiving funding from pharmaceutical companies did show significant effects. Similarly, high-quality studies of “collagen supplements” revealed no significant effect in all categories, while low-quality studies revealed a significant improvement in elasticity.
No evidence that collagen supplements improve skin health
First, “collagen supplements” don’t contain collagen – they contain hydrolyzed collagen, otherwise known as amino acids. Moreover, most “collagen supplements” contain many ingredients other than the hydrolyzed collagen. A new meta-analysis by scientists at two major universities casts doubt on the effectiveness of “collagen supplements” for improving signs of skin aging, raising questions about the role of industry-funded research in shaping health and wellness trends. As the authors conclude, “There is currently no clinical evidence to support the use of “collagen supplements” to prevent or treat skin aging.” This is nothing new. Many studies in the pharmaceutical and supplement industries are flawed, frequently using fraudulent data, to sell their products that actually don’t work.
Often times, pharma and supplement companies hire ghostwriters to perform and write-up the study. Then the company finds a physician who will put their name on the byline. The physician didn’t have anything to do with the study and had nothing to do with writing the study. Ghostwriting is a big problem, and growing. For example, “first author” of a medical paper on Vioxx, Jeffrey Lisse, M.D., has said in an interview that “Merck designed the trial, paid for the trial, ran the trial…Merck came to me after the study was completed and said, ‘We want your help to work on the paper.’ The initial paper was written at Merck (ghostwritten), and then it was sent to me for editing.” In other words, Mr. Lisse was not an author of the study but was paid to pretend he was. Not only were his actions immoral, they were dangerous. Vioxx was later removed from the market because it significantly increases cardiovascular adverse events – people died from heart attacks. As physician Adriane Fugh-Berman, M.D., professor at Georgetown University School of Medicine and PharmedOut, has said about ghostwriting, “But there’s also the fact that this is so common that it’s not considered unusual. There’s no shame attached to it.” The point here is that many studies of drugs and supplements are flawed, some are fraudulent, and the studies of collagen supplements seem to be highly flawed.
Collagen and its importance to skin function and health
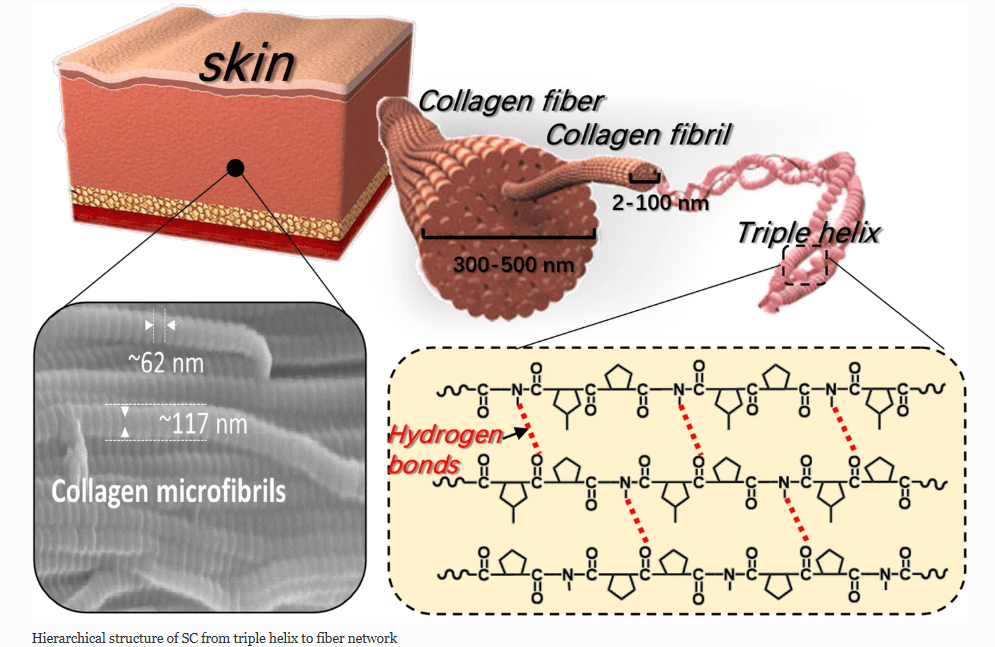
From: Han et al (2021) Recent advances in skin collagen: functionality and non-medical applications
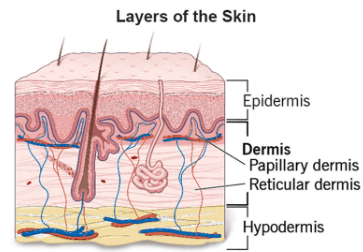
Skin Collagen is a scleroprotein (not water soluble) and a major structural protein found throughout the body, including in skin (Fig. 2), hair, nails, tendons and bones. Much collagen in the body, including in the skin, is long-lived. One estimate of human skin collagen half-life suggested 14.8 years. Specifically, type I and type III collagen are found in abundance in the skin. Elastic fibers also play an important structural role within the dermis. Elastic fibers are composed of elastin and fibrillin microfibrils. In contrast to collagen, the biochemical configuration of elastin allows for gliding, stretching, and recoiling of fibers. The reticular dermis comprises thick elastic fibers. Two subtypes of elastic fibers are noteworthy: elaunin and oxytalan fibers. Elaunin fibers are horizontally arranged elastic fibers found near the junction of the papillary and reticular dermis. Oxytalan fibers are perpendicular elastic fibers found in the papillary dermis. Fibers work alongside substances like glycosaminoglycans (GAGs), such as hyaluronic acid, to maintain skin elasticity, volume, and moisture. While the body naturally produces collagen using amino acids from foods like beans and tofu, production declines with age and can be further reduced by sun exposure and poor diet.
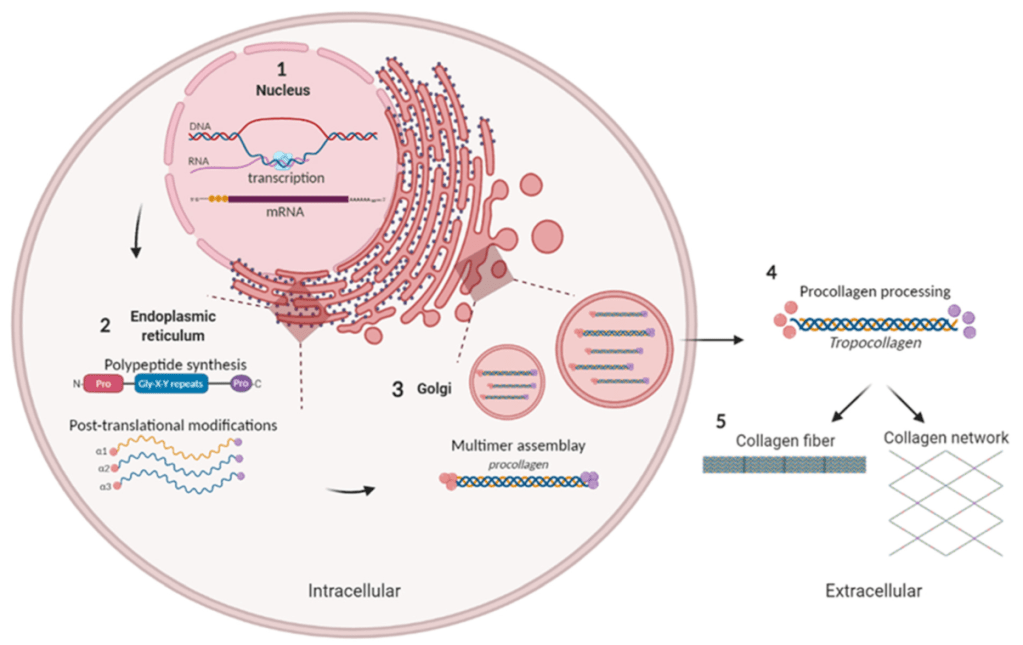
From Alcaide-Ruggiero et al (2021). Schematic representation of collagen biosynthesis. (1) Gene transcription. (2) Formation of α-chains. (3) Formation of triple helix procollagen and secretion into extracellular space. (4) Procollagen processing and formation of tropocollagen (non-soluble form of collagen). (5) Association of tropocollagen molecules to form collagen structures.
Many forms of collagen, such as type I, are abundant through a range of tissues and are fundamental structural building blocks. Type I collagen is the main component of fibrils that provide tissues with tensile strength. Type I collagen is a heterotrimeric protein assembled from the two α1(I) and one α2(I) polypeptides when they fold into a triple helix. After secretion of procollagen into the extracellular space, the terminal domains are removed by proteolytic cleavage and the rodlike triple helices of the central domain polymerize into fibrils and are covalently cross-linked.
Type I collagen is the most abundant collagen type in the skin, accounting for 80–85% of the dermal ECM. Other subtypes, like type III and type V collagen, can be found in skin, but type I collagen is fundamental to skin structure; it supplies considerable tensile strength and helps to determine the structure and durability of the dermis. As skin ages, there is a progressive loss, damage, and fragmentation of dermal collagen fibrils, leading to reduced skin thickness and biomechanical strength.
Type I collagen is also one of the longest-lasting of the long-lived protein in humans, and is a major fibrillar component of connective tissues such as skin, bone, and tendons. It has a triple-helix structure composed of two α1 chains encoded by the Collagen type I alpha 1 chain (COL1A1) gene and one α2 chain encoded by the Collagen type I alpha 2 chain (COL1A2) gene. Among these, COL1A1 expression has been identified as a biomarker of skin aging, as its levels decline with age. This organization of collagen along with other fibrils and matrix molecules endows connective tissues with mechanical strength and elasticity.
Collagen genetics
The COL1A1 gene provides instructions for making part of type I collagen. A component of type I collagen called the pro-α1(I) chain is produced from the COL1A1 gene. Collagens begin as rope-like procollagen molecules that are each made up of three chains. Type I collagen is composed of two pro-α1(I) chains and one pro-α2(I) chain (which is produced from the COL1A2 gene).
The triple-stranded procollagen molecules are processed by enzymes in a series of steps inside and outside the cell to create mature collagen. The collagen molecules then arrange themselves into long, thin fibrils that form stable interactions (cross-links) with one another in the spaces between cells. The cross-links result in the formation of very strong type I collagen fibers.
Industry funded pharmaceutical and supplement studies are often flawed
While collagen drinks, supplements, topical products and even prescription pharmaceuticals have gained market traction for their promised skin benefits, the stark difference between the overall results and the subgroup findings underscores how industry funding and study quality can sway outcomes, a longstanding concern in nutrition, pharmaceutical, and supplement research. Sadly, many medicines and supplements don’t work, including those for dermatological use. Allen Rogers, PharmD, worldwide vice president of genetics at Glaxo SmithKline, is reported on the front page of the Independent (8 December, p 1) as saying: “Our drugs don’t work on most patients.”
Confounding these studies of collagen, most of the trials used commercially available supplements that contained more than hydrolyzed collagen (amino acids), including vitamins, minerals, antioxidants, coenzyme Q10, hyaluronic acid and chondroitin sulfate were among the additional ingredients.
Too much amino acid consumption is bad for health, including heart health
Scientists have discovered a molecular mechanism by which excessive dietary protein could increase atherosclerosis risk. For example, amino-acid-mediated mammalian target of rapamycin (mTOR) signalling in macrophages has been implicated in the pathogenesis of ischemic cardiovascular disease. Specifically, ingestion of protein or amino acids in excess of ∼22% of dietary energy requirements drives atherosclerosis.
Further, an unbalanced, unnatural increased intake of one or more amino acids can cause imbalance in amino acid concentrations in the body, increase concentrations of its metabolites, and affect the transport of a group of amino acids into cells due to competition for a carrier at the cell membrane. The phenomenon of carrier competition can affect absorption of other amino acids in the gut and subsequently their appearance in the blood, transport across the blood-brain barrier, and supply for protein synthesis. Proteinopathies may result, leading to degenerative disorders. For example, too much leucine consumption can decrease autophagy in the brain. Autophagy, a cellular process of waste and debris removal, is known to decrease proteinopathy, and therefore too much leaucine may potentially lead to the buildup of toxic metabolites and neurodegeneration.
Summary
Skip the supplements made from hydrolyzed collagen. Eating a variety of plant-based protein sources—such as beans, soy, legumes and quinoa—means your body will have the amino acids it needs to make collagen, while also providing Vitamin C and antioxidants, all of which are important to the health status of the skin, particularly collagen formation and protection of the long-lived collagen.