John Sanderson, whose medical license was revoked for sexual misconduct and repeated negligence, has sold his company, AnteAge, to a private equity company. Now that the PE company has taken over, their marketing people have invented a new word, “Biosome.” for what is called by scientists, a “liposome.”
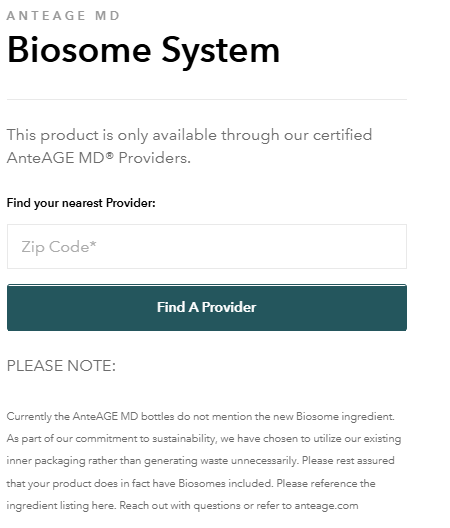
How do we know the Private Equity guys who own AnteAge are using fake technology? Look at the staement from their website: “Currently the AnteAGE MD bottles do not mention the new Biosome ingredient. As part of our commitment to sustainability, we have chosen to utilize our existing inner packaging rather than generating waste unnecessarily. Please rest assured that your product does in fact have Biosomes included. Please reference the ingredient listing here. Reach out with questions or refer to anteage.com.”
Here’s the statment from their web:
How Do We Know They’re Faking It
If there were actually something new in the bottle, they would have to, by law, relable the product. In other words, because they have only changed their marketing hype, and not the product’s technology, they don’t need to make any changes to the bottle labeling – specifically the bottle’s listing of ingredients. The label and the ingredients remain the same and the only thing changing is what they call the product. There’s no validation in peer-reviewed literature or patent filings confirming a unique mechanism under the name “Biosome.” Rather, it’s just marketing hype, or as some would call it, BS.
So what’s in the bottle? Liposomes. Look at the ingredients on the bottles with the new, fake technology. The list includes “Phosphatidylcholine.” Guess what are made with Phosphatidylcholine. Answer – liposomes! So now AnteAge is calling liposome, you guessed it, Biosomes. This is Private Equity at work. Say anything, do anything, for profit.
Here’s the bottle saying “Biosomes”:

And here’s the bottle’s ingredient list for the “Serum”:
Serum Ingredients:
Water (Aqua), Human Bone Marrow Stem Cell Conditioned Media, Cetyl Ethylhexanoate, Niacinamide, Dimethyl Isosorbide, Polyacrylate-13, Glycerin, Hydrolyzed Myrtus Communis Leaf Extract, Butylene Glycol,
Carbomer, Polysorbate 20, Palmitoyl Tripeptide-1, Palmitoyl, Tetrapeptide-7, Polyisobutene, Benzyl Alcohol, Salicylic Acid, Sorbic Acid, Sorbitan Isostearate, Carnosine, Ilex Paraguariensis Leaf Extract, Maltodextrin,
Disodium EDTA, DOTAP, DSPC, DSPE, DSPE PEG, Sodium Chloride, Disodium Phosphate, Potassium Phosphate, Potassium Chloride, Phosphatidylcholine, Phosphatidylserine, Sphingomyelin, Cholesterol, Mannitol,
Trehalose, sh-Oligopeptide-33, sh-Polypeptide-58, sh-Polypeptide-5, sh-Polypeptide-2, sh-Polypeptide-67, sh-Polypeptide-66, sh-Polypeptide-10, sh-Polypeptide-3, sh-Polypeptide-62, sh-Polypeptide-14,
sh-Oligopeptide-2
Bottome line. Private equity is ruining many things and now they’re lying to the public about skin care ingredients.
If you’d like to read about the science of exosomes and liposomes, you can read my 30 page academic book chapter, peer-reviewed, that I published in 2016 with Elsevier, called “Exosomes: smart nanospheres for drug delivery naturally produced by stem cells.“