Based on many recent scientific studies of using live, symbiotic bacteria in a topical application, NeoGenesis has launched a new probiotic, topical skin care product (MB-2) and will be launching MB-3 soon. I briefly explain some of the science for using topical probiotic products in this post.
I’ve been publishing about (Maguire and Maguire, 2017) and developing topical probiotic products (MB-1 was launched in 2015) for well over a decade. The data for topical symbiotic bacteria colonizing and benefiting the skin are rapidly accumulating. For example, topically applied Lactobacilli have been found to temporarily colonize the skin and to directly compete with skin pathogens through adhesion inhibition, production of antimicrobial metabolites, and by influencing pathogen metabolism. The competitive anti-pathogenic action of Lactobacilli has been described mechanistically for common skin pathogens, such as Staphylococcus aureus, Cutibacterium acnes, and Candida albicans (DeLanghe et al, 2021). Recently, studies of live Lactobacillus crispatus (LBC) demonstrated benefit to the skin when compared to inactivated LBC biomass, stimulating collagen in vitro. Moreover, the live LBC was stable in formulations not containing antimicrobial preservatives and was found to improve dermis density and wrinkle appearance in vivo.
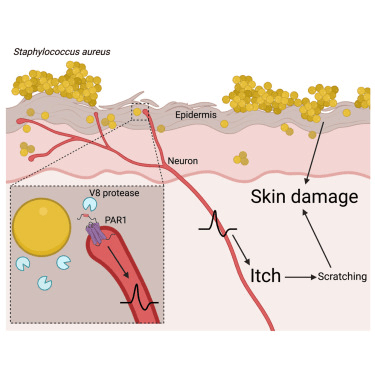
Microbes and human cells have co-evolved for billions of years, through which they have been exposed to many types of molecules produced by each other and acting in bidirectional signaling pathways (Wu et al, 2025). For example, Lactobacilli have an immunomodulatory capacity associated with a reduction in excessive skin inflammation (Delanghe et al, 2021). Their influence on the immune system is mediated by bacterial metabolites and cell wall-associated or excreted microbe-associated molecular patterns (MAMPs). Lactobacilli acting as immune modulators associated with a reduction in excessive skin inflammation exert their influence on the immune system by secreting many bacterial metabolites, a type of postbiotic (this is a term I introduced in 2019; Maguire and Maguire, 2019), along with the cell wall-associated MAMPs that are not released but integrated into the cell of the bacterium. In addition, Lactobacilli can also enhance the skin barrier function, which is often disrupted as a result of infection, trauma, or in inflammatory skin diseases such as eczema and psoriasis. Lebeer et al (2022) found that the Lactobacillis species L. crispatus, L. iners, L. gasseri, and L. jensenii, all still belonging to the genus Lactobacillus strictu sensu, have a broader human adaptation to stratified epithelium than merely the human vaginal epithelial cells, based on their association with healthy skin. In other words, Lactobacilli colonize the skin just as they do in other epithelial tissues. However, these colonies of bacteria on the skin can be disrupted by a number of extrinsic and intrinsic factors, such as harsh chemicals and aging. For example, aged skin contains significantly fewer L. crispatus (a beneficial symbiont) than young skin. Let’s now breiefly look at how symbiotic bacteria benefit the skin through quorum sensing and the release of post-biotic molecules, including molecules that will inhibit pathogenic bacteria such as certain strains of Staphlacoccus aureus.
Quorum Sensing and Post-Biotic Release

Mechanisms of quorum sensing is different for gram-positive versus gram-negative bacteria. Regardless, quorum sensing molecules (AIP or QS molecules) can work within species or on other species to control growth. This is an important means by which symbiotic bacteria, such as B. subtilis, can inhibit pathogenic bacteria such as S. aureus.
As bacteria grow, they secrete and sense signaling molecule in the surrounding environment. By detecting variations in the concentration of these signal molecules, bacteria can modulate the expression of related genes, thereby regulating associated behaviors. Consequently, interfering in bacterial QS signaling to either promote or inhibit the development of lactic acid bacteria (LAB) biofilms holds substantial significance in terms of enhancing skin immunity, promoting skin health.
Quorum sensing allows bacteria to communicate and coordinate collective behaviors by sensing population density through chemical signals, or autoinducers. While primarily species-specific, interspecies communication also occurs when different bacteria produce or detect shared autoinducers like autoinducer-2 (AI-2), a “universal” signal molecule used by many species. This interspecies communication can lead to either cooperation or competition, influencing functions such as biofilm formation, virulence, and resistance against other microbes.
For example, colonization of the skin by Staphylococcus aureus is associated with exacerbation of atopic dermatitis (AD). Proteases and phenol-soluble modulin α (PSMα) secreted by S. aureus leads to endogenous epidermal proteolysis and skin barrier damage that promotes inflammation (Williams et al, 2019). Other species of bacteria residing on normal skin can produce autoinducing peptides that inhibit the S. aureus agr system, in turn decreasing PSMα expression. A number of bacteria types, such as Bacillus subtilis (it secretes lactic acid), can quorum sense (Spacacan et al, 2020) and react to the S. aureus overcolonization by inhibiting the S. aureus through disruption of their QS system (Leistikow et al, 2024).
Quorum-sensing systems in the skin can be divided into two paradigmatic classes: LuxI/LuxR–type quorum-sensing systems in Gram-negative bacteria and oligopeptide/two-component–type quorum-sensing circuits in Gram-positive bacteria. All of this is very complicated, relaizing that bacteria have elaborate chemical signaling systems that enable them to communicate within and between species is only recently been explored and the field is emerging quickly. Based on our current knowledge, I’ve developed two new products, MB-2 and MB-3, using symbiotic, live bacteria known to perform QS or interfere with QS in other bacteria strains and promote skin benefits, including reduced inflammation and barrier function rebuild.
Interspecies Quorum Sensing Fosters Both Competition and Collaboration
To be clear, quorum sensing between different bacterial species occurs as well. For example, some species cannot produce their own autoinducers, but have receptors for the autoinducer molecules of other species, allowing them to sense and respond to others in their environment. Like human behavior, bacteria behavior operates on a continuum of individualism and collectivism. This quality can breed conflict, but also collaboration and interspecies quorum sensing can take both forms. In other words, the good guys, the symbiotic bacteria, can work together through quorum sensing among themselves (intraspecies quorum sensing) to inhibit the bad guys, the pathogenic bacteria, through interspecies quorum sensing. The good guys can be fighting some bacterial species, such as S. aureus, that use quorum sensing to enhance each other’s virulence.
Let’s now look at the five symbiotic bacteria that are contained in MB-2 and MB-3
Lactobacillus plantarum
Lactobacillus plantarum treatment reduced wound bacterial load, neutrophils, apoptotic and necrotic cells, modified IL-8 production and induced wound healing (Peral et al, 2010). When topically applied to a disease skin model for acne, L. plantarum induced a significant reduction in viability of virulent bacteria phylotypes, lipid production, and modulated inflammatory markers (Podrini et al, 2023). Further, L. plantarum whole cultures promote tissue repair, and this bacterium may also improve the healing of diabetic wounds in rats through the regulation of inflammatory cytokines (Ishi et al, 2023). In a study of 23 subjects, topical L. plantarum in a cream formulation was found to benefit skin aging properties, including TEWL, barrier function, and wrinkles (Elvebakken et al, 2023).
Lactobacillus crispatus
An oily suspension containing Lactobacillus crispatus and Lacticaseibacillus paracasei was found to benefit Seborrheic dermatitis (Truglio et al, 2024). In a study of 29 women with topical application of L. crispatus, the density of the sub-epidermal zone significantly increased vs baseline by 11% and of the dermis by 6% (+5% vs placebo). As I mentioned in the introduction,, studies of live Lactobacillus crispatus (LBC) demonstrated benefit to the skin when compared to inactivated LBC biomass, stimulating collagen in vitro. Moreover, the live LBC was stable in formulations not containing antimicrobial preservatives and was found to improve dermis density and wrinkle appearance in vivo.
Bacillus subtilis
Topical application of live Bacillus subtilis has been found to reduce the number of pathogenic bacteria in skin, including S. aureus (Moskovicz et al, 2021; Piewngam et al, 2023). Topical application also helps to reduce acne breakouts (Ma’or et al, 2023). B. subtilis is being developed for drug delivery for a number of reasons (Montgomery et al, 2024). It may have advantages over other candidate bacteria as a platform for drug delivery to the skin because of its safety profile and genetic tractability. It is found in the skin microflora and is metabolically active on the skin. It is nonpathogenic and has natural antimicrobial properties against pathogenic staphylococci and fungi. B. subtilis has generally regarded as safe status from the FDA, and multiple B. subtilis probiotic products as well as a genetically modified strain of B. subtilis are currently commercially available. Further, an important characteristic of B. subtilis is that it is commonly used in biotechnology for the production of proteins, vitamins, and antibiotics because of its efficient protein secretion system, and ease of cultivation, factors that mean it can work well when topically applied to the skin as a probiotic.
Bacillus coagulans
LactoSporin, a metabolite of Bacillus coagulens, cream topically applied for 10 weeks resulted in a significant reduction in visibility of wrinkles around crow’s feet, nasolabial folds, frown lines, and facial fine lines compared to baseline and placebo by dermatological and Antera imaging assessments (Majeed et al, 2023). . Optimal conditions for growth include a temperature range of 30–50°C and a pH level of 5.5–6.5, matching the surface of the skin. This bacterium exhibits weak adhesion to epithelial cells, which prevents long-term colonization, but allows temporary colonization and yielding positive effects.
Lactoccus lactis
Various strains of L. lactis have recently been reported to induce anti-inflammatory activity in vitro (Luerce et al, 2014). Administration of L. lactis LB 1022 improved clinical AD symptoms, decreased serum IgE and suppressed the Th2 cytokines secretion, such as IL4, IL-13, and TSLP in blood, which are factors found to be elevated by AD. Similarily, oral L. lactis LB 1022 may have a protective effect against AD by reducing high IgE serum levels and Th2-related responses that arise from an imbalance in the gut microbiota. Topical application of L. lactis is likely to have similar effects on AD, but given the likely lower colonization levels when topically applied, requires more frequent dosing to achieve similar positive results. It is also possible that L. lactis ferments glycans on the surface of the skin, thus producing beneficial lactic acid that may then be fermented into beneficial short chain fatty acids which then regulate the immune system and reduce inflammation.
Summary
As you can read here in the studies I’ve mentioned, there is much accumulating evidence for the benefits of the 5 types of symbiotic bacteria that I’ve chosen to include in our NeoGenesis MB-2 and MB-3 products. Working with a number of dermatologists in the USA, we’ve had remarkable postive results in compromised, inflammatory skin conditions where MB-2 (bacteria in an occlusive base) serves those conditions with interupted barrier function, such as atopic dermatitis and MB-3 (in a non-occlusive oil base) serves those conditions with a more intact barrier and oily and pustule-prone skin.