Inflammation is for fighting pathogens, and it is destructive to the skin. Inflammation is not needed to regenerate the skin or induce collagen production, and actually slows and impedes the healing process. There is a potent and safe means to inhibit the inflammatory pathways and promote regenerative healing in the skin -S2RM Technology- stem cell released molecules from skin derived adipose mesenchymal stem cells and fibroblasts.

From: Liu et al 2017
I continuously hear that inflammation is needed to heal the skin and to produce collagen and rebuild the matrix. This is false, and I’ll tell you why, and tell you the differences in the two healing processes, i.e 1. non-inflammatory regenerative healing versus, 2. inflammatory reparative healing.
Once we’ve exited the sterile or nearly sterile womb, most postnatal wounds heal through reparative healing, which is a complex biological process involving cells, signaling molecules such as growth factors and other cytokines, and the extracellular matrix (ECM). Wound healing is simplified and described as occurring in four overlapping, highly coordinated stages: hemostasis, inflammatory, proliferation, and remodeling. In the womb, where there are no pathogens, inflammation is not needed to fight infection – there’s no pathogens present to infect the skin. In the fetus, the immune system in the skin is only beginning to develop and is not robust, and platelets that normally rush into wounded skin are not yet fully developed, and the blood cells are being produced in the liver and not in the bone marrow. Wound healing in the fetus is vastly different from that in the adult. Adipose mesenchymal stem cells (AMSCs) arise later in fetal development in order to control and resolve the newly formed inflammatory mechanisms in the skin that are important in the adult to fight infection using an inflammatory response.
Whether it is macrophages or T cells, including γδ T-cell subsets, or other immune cells, it is the AMSCs that serve to calm the early-onset inflammation by polarizing the immune cells from an inflammatory type to an anti-inflammatory, pro-regenerative type. This is in contrast to bone marrow mesenchymal stem cells (BMSCs), which are a major source of IL-7, thus producing inflammation, and playing a pathological role in the maintenance of inflammatory CD4 memory T-cells that are involved in autoimmunity and chronic inflammation. BMSCs and the molecules they release also have oncogenic potential, another reason why they are an inferior choice for therapeutic development.
Regenerative Non-Inflammatory Healing and Reparative-Inflammatory Healing
Fetal wounds heal in utero through regenerative healing; postnatal microenvironments with an attenuated inflammatory response, such as the oral mucosa, also heal with regenerative characteristics, including a reduced immune response and scarring. Regenerative healing occurs in a manner similar to the same four stages of reparative healing, with some key differences. The key difference is that compared with reparative healing, the inflammatory response in regenerative healing is attenuated. Many of the cells involved in both innate and adaptive immunity, such as mast cells, macrophages, and neutrophils, are not yet differentiated or are not responsive to the wound where regenerative healing occurs. Therefore, levels of inflammatory cytokines and chemokines are reduced or absent in regenerative healing.
Increased expression of anti-inflammatory cytokine IL-10 in postnatal regenerative healing helps decrease the inflammatory response. Adipose mesenchymal stem cells are a key source of the IL-10 secreted into the skin, and thus promoting regenerative healing. A number of studies suggests that IL-10 not only indirectly modulates fibrosis via its anti-inflammatory properties but may also stimulate fetal-like fibroblast behavior and thus fetal-like ECM production. If scar tissue is to be of normal structure, regenerative healing must take place. The secretion of IL-10 from AMSCs is key to inducing regenerative healing in adult skin. One mechanism to explain the ability of IL-10 to inhibit inflammation is that it inhibits NF-κB activity by inhibiting nuclear translocation of NF-κB by blocking IκBα degradation in response to TNF stimulation.
Collagen Production in Wound Healing – Inflammation Degrades Collagen, Not Produce It
Collagen production in the skin to aid in healing, is mainly derived from fibroblasts, but also by keratinocytes. Fibroblasts have evolved to regulate their synthesis of collagen and other extracellular matrix proteins in response to mechanical tension. Fibroblasts are also induced to secrete collagen by the molecules released from AMSCs. It’s not inflammation that stimulates the production of collagen. Tissue damage caused by inflammation from an infection or an autoimmune disease triggers degradation of collagen in the extracellular matrix (ECM), which further enhances inflammation. So inflammation is degrading collagen, not producing it. Also know that dermal collagen has a half-life of about 15 years, a very long-lived protein, a feature that predisposes collagen to accumulate lesions such as advanced glycosylation end products (AGE), which have damaging effects on the molecules they bind. So with much collagen in the skin lasting for decades, accumulating damage through inflammation is occurring. The secretome from AMSCs can protect these long-lived collagen proteins from inflammatory damage, while also helping to replace damaged collagen.
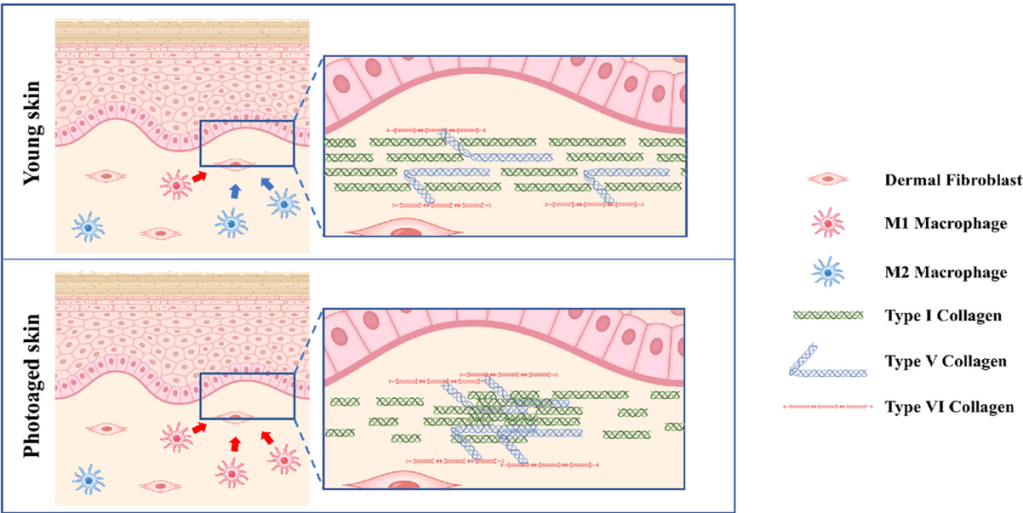
Non-Inflammatory Immune Cells, M2 Macrophages are Anti-inflammatory and Pro-Regenerative
Often, the delay in tissue healing results from the inflammatory phase of the wound healing. Non-healing wounds result from chronic inflammation, characterized by an overload of inflammatory immune cells, inflammatory cytokines, and proteolytic enzymes. Chronic wounds share certain common features, including excessive levels of proinflammatory cytokines, proteases, ROS, senescent cells, persistent infection, and a deficiency of stem cells and their released molecules that are often also dysfunctional. Chronic wounds are defined as wounds stalled in a constant and excessive inflammatory state. For example, much evidence has revealed that chronic wounds are closely associated with impaired phenotype transition of pro-inflammatory macrophages (M1) to anti-inflammatory phenotypes (M2) in wounds. The secretome from AMSCs biases the macrophage phenotype from an inflammatory M1 to an anti-inflammatory, pro-regeneration M2 phenotype, and greatly aids in wound healing. An example of the pro-healing effects is that M2 macrophages induced the expression of the proteins required for the assembly of collagen fibrils, and macrophages themselves secrete some forms of collagen. A shift towards M2 in the M1/M2 balance improves not only the quantity but also the quality of collagen fibrils, leading to a non-fibrotic scar. M2 macrophages induce the expression of the proteins required for the assembly of collagen fibrils,
From: Horiba et al (2023)
In wounds, the continued infiltration of pro-inflammatory immune cells and production of pro-inflammatory molecules attract additional inflammatory immune cells, exacerbating the inflammation. Thus, inflammation is preventing wound healing. If you think inflammation is needed to clear debris in a wound, including “sterile inflammation,” think again. During the resolution of inflammation, macrophages are predominantly polarized to an M2 phenotype (non-inflammatory), which can suppress proinflammatory cytokine production, clear debris, and restore tissue homeostasis. Yes, M2 macrophages are phagocytic – meaning they eat debris. Again, wounds don’t need inflammation to heal, whether it’s an infected wound, or “sterile inflammation” where debris is present without infection.
The Inflammatory NK-kB Pathways Are Pro-Inflammatory and Impede Wound Healing
Recent studies have found the genes and pathways involved in the induction of inflammation, called, NF-kB, and that these genes and pathways are also involved in aging and many disease processes. The NF-kB pathways underlying inflammation, diseases, and aging (inflammasome) are different from the genes and pathways that are activated during injury and responsible for regenerative healing.
Leung et al (2013) at Stanford did a nice study separating out the two pathways involved in adult healing, i.e. the NF-kB pathways. They found that hypochlorite (HOCl) reversibly inhibited the expression of CCL2 and SOD2, two NF-κB–dependent genes. In radiation dermatitis, topical HOCl (aka Bleach) inhibited the expression of NF-κB–dependent genes, decreased disease severity, and prevented skin ulceration. Additionally, skin of aged HOCl-treated mice acquired enhanced epidermal thickness and proliferation, comparable to skin in juvenile animals. In other words, inhibiting inflammation helped to heal the skin when injured through irradiation or aging.
Platelets, Bone Marrow Mesenchymal Stem Cells, and Their Molecules Induce Inflammation
This is why you don’t want to use a platelet extract or PRP on your skin, because platelets and their molecules induce inflammation. It’s also why you don’t want to use bone marrow mesenchymal stem cell derived molecules because they too are pro-inflammatory and can induce, through IL-7, autoimmunity and tissue destruction. Both of these cell types only appear transiently in open wounds through the blood supply and serve to induce inflammation to fight infection, and to induce high rates of fibrotic scarring to close the wound rapidly (see Maguire, 2022).
Adipose Mesenchymal Stem Cells and Their Molecules Reduce Inflammation
There’s another safe and effective means to inhibit the inflammatory pathways associated with NF-kB, and that is the secretome from AMSCs, which is contained in the S2RM Technology of NeoGenesis. González-Cubero et al (2022) in Spain found that in inflamed human cells from connective tissue, like that found in the skin, when exposed to the molecules released from AMSCS, NF-κB activation was blocked. Thus, the secretome from AMSCs blocked inflammation by blocking the NF-kB pathways. Adipose mesenchymal stem cells and their released molecules act in many other ways in addition to inhibiting NF-kB to reduce inflammation.
Chronic Inflammation
So the adipose mesenchymal stem cells reduce inflammation and this is critical for autoimmunity. Tregs control other T cells from over activating and causing damaging inflammation. It is important to keep Treg cell functioning because they tend to lose their regulatory capacity under chronic severe inflammatory conditions. If a patient’s Treg cell function is compromised or defective, their immune system can become excessively activated, leading to systemic autoimmune inflammation. So AMSCs controlling inflammation in turn controls Treg cell function and reduces T cell mediated autoimmune inflammation.
Summary – Inflammation is Unwanted in Wound Healing and Adipose Mesenchymal Stem Cells Deactivate Inflammation and Activate Regenerative Healing
In summary, inflammation does not heal the skin and does not produce collagen. Inflammation is unwanted in the skin unless their is an infection and the pathogens need to be destroyed. Unfortunately, in the process of killing the pathogens, the inflammation also damages your cells and connective tissues. To effectively and safely decrease inflammation and activate regenerative wound healing, use NeoGenesis Recovery which is loaded with the molecules released from AMSCs that reduce inflammation and activate pro-healing regenerative mechanisms.