Adipose mesenchymal stem cells (ADSCs) have evolved to arise in the skin during the third trimester of fetal development. These cells arise just before birth so that they can be present following birth to tampen inflammation that may arise in the baby’s new hostile, non-sterile environment where the skin is under constant insult from injuries, toxins, UV, antigens, and pathogens. It’s why ADSCs and the molecules they release are preferred over, 1. bone marrow mesenchymal stem cells and platelets, which serve to induce inflammation and rapid fibrotic scarring, and 2. over umbilical cord mesenchymal stem cells, that have evolved to operate in the sterile conditions of the womb to form the cord, which is unlike skin structure and function, and since it’s a sterile environment, not dampen inflammation which is unneeded and doesn’t happen in the sterile environment where infection can’t happen. The molecules released from ADSCs are the safest and most effective stem cell released molecules to use as skin therapeutics.
Scientist think teleologically often. It’s one of the ways we reason through the discovery and invention of phenomenon. Teleology is relating to or involving the explanation of phenomena in terms of the purpose they serve rather than of the cause by which they arise. In other words, teleology or finality is a branch of causality giving the reason or an explanation for something as a function of its end, its purpose, or its goal, as opposed to as a function of its cause. Why is this thing present, what is it doing?
Adipose mesenchymal stem cells (ADSCs) have evolved to arise in the skin during the third trimester of fetal development and to be present throughout adult life. These cells arise just before birth. So the teleological questions are, why do they arise just before birth, and what are the doing in the adult skin during a person’s lifetime?
Teleologically thinking, the ADSCs are present following birth to tampen inflammation that may arise in the baby’s new hostile, non-sterile environment that presents after birth. The ADSCs arise as tissue specific stem cells in the skin that has developed during the third trimester. The stem cell niche of the skin will help to direct these ADSCs to develop in a manner that is tissue specific and serves to resolve inflammation in that adult skin. This sort of tissue specific development of the ADSCs doesn’t happen in the bone marrow or the umbilical cord, for example. Following birth, the skin is under constant insult from traumatic injuries, toxins, antigens, UV, and pathogens. Those are signals for inflammation. When the skin is compromised by these factors, evolution has given the skin an inflammatory response to fight associated infection. Any of these factors can lead to barrier disruption and an eventual infection, and the inflammatory response is the key to fighting infection. But inflammation is damaging. Not only does infection fight invading pathogens, inflammation also damages our own cells and tissues.
So inflammation has to be tampened, otherwise, if it is prolonged, necroinflammation ensues and our tissues become necrotic or otherwise damaged. Without inflammation being reduced, the damaging inflammatory pathways cause more inflammation and scale-up the damage. And what is present in adult skin to resolve inflammation? It’s the adipose mesenchymal stem cells (ADSCs) and the molecules that they release. In this case, the molecules from ADSCs can help the healing process by a number of mechanisms, including angiogenesis and reducing inflammation. The molecules from ADSCs induce an anti-inflammatory pro-regenerative state in the skin. Diabetic ulcers are example, where the necrotic tissue, such as Necrotizing fasciitis, has to be removed to reduce the inflammation. In these conditions, the ADSCs are no longer present at the site of open wound, and inflammation is hard to control. Addition of ADSC secretome facilitates the healing of the diabetic ulcer through a number of mechanisms, including the reduction of inflammation.
ADSCs are preferred over, 1. bone marrow mesenchymal stem cells and platelets, which serve to induce inflammation and rapid fibrotic scarring, and 2. over umbilical cord or placental mesenchymal stem cells, that have evolved to operate in the sterile conditions of the womb to form the cord, which is unlike skin structure and function, and since it’s a sterile environment, not dampen inflammation.
There’s much hype about cytokines from bone marrow mesenchymal stem cells. I’ve previously blogged about how bad these BMSC molecules are for the skin. Let’s quickly consider inflammation and the stem cells used by AnteAge to make their products: Bone Marrow Mesenchymal Stem Cells (BMSCs), and the molecules they release, prolong and enhance inflammation by increasing survival and function of neutrophils (Castella et al, 2011). Under hypoxic conditions, which induces the activation of TRL4, BMSCs secrete pro-inflammatory factors and decrease the polarization of macrophages from the M1 to M2 phenotype (Faulknor et al, 2017; Waterman et al, 2010). Therefore, BMSCs cultured in normal hypoxic conditions in the laboratory are secreting pro-inflammatory factors and when administered to wounded skin will induce inflammation by recruiting neutrophils and M1 type pro-inflammatory macrophages. When you put AnteAge on your skin, these are the pro-inflammatory molecules damaging your skin.
Safety and efficacy considerations: ADSCs preferred Over BMSCs
I’m asked frequently about the safety of using the molecules from ADSCs, so I’ll address it here. When addressing safety and efficacy concerns of stem cells, we must consider tissue-specific stem cells, first described by Dr. Elly Tanaka, a professor of science at the IMP in Vienna. Choosing the appropriate stem cell type to match the condition to be treated is critical not only to efficacy, but most importantly, safety of the therapeutic. Beyond the genetic and epigenetic factors that influence stem cell phenotype as embryonic stem cells differentiate into somatic stem cells, the immediate niche of the stem cell will have profound influence on the cell’s phenotype. If your wanting to regenerate skin, then use tissue specific stem cells from the skin. ADSCs and their secretome is efficacious and safe. Even ADSCs from cancer patients can been safely used for therapeutic purposes.
We don’t use umbilical cord mesenchymal stem cells (UMSCs) because they are not tissue specific to the skin, and they didn’t evolve to work in adult tissue where inflammation needs to be inhibited. Bone marrow mesenchymal stem cells (BMSCs) do appear in the skin, but only transiently in the skin during open wounds to close the wound quickly (yielding fibrotic scarring). induce inflammation (destructive to tissue), and cause high rates of proliferation (pro-oncogenic). If you think about it, the BMSCs appear transiently during an open wound to fight infection by inducing inflammation, and closing the open wound quickly by hyper-proliferation of cells. BMSCs and their released molecules didn’t evolve to be present in the skin for long periods of time – only transiently. Applying BMSC molecules for an extended time will induce too much inflammation and too much proliferation, leading to long term inflammation, fibrotic scarring, and a pro-oncogenic state.
Beyond their suboptimal efficacy profile, I’ll briefly explain some of the mechanisms underlying our choice of not using BMSCs because of a poor safety profile. The complexity of the bone marrow (BM) niche can lead to many stem cell phenotypes, whether we consider hematopoietic stem cells (HSCs) or bone marrow mesenchymal stem cells (BMSCs). Here I will discuss the properties of BMSCs, not HSCs. Because of the complexity, many BMSC phenotypes exist, including disease causing phenotypes that are varied and hard to distinguish – a part of the problem in using BMSC for therapeutic development. This complication, unlike that for ADSCS, includes recirculated cells, particularly recirculated cancer cells. Once a tumor cell disseminates into the BM, the cancer cell often displays phenotypic characteristics of BMSCs rendering cancer cells difficult to distinguish from BMSCs. BM is a site of BMSCs that may differentiate into HSCs [113] and recirculating blood cells that may differentiate into BMSCs [114,115]. BMSCs are also found outside of the niche in peripheral blood [116] and home into sites of injury [117] and cancer tissue where they are educated into becoming a pro-cancerous phenotype [118]. Recirculated melanoma and myelogenous leukemia cells [119] in BM interact with BMSCs to change the phenotype of the BMSC to one that is cancer promoting by enhancing their proliferation, migration, and invasion and altering the production of proteins involved in the regulation of the cell cycle [120]. Indeed, melanoma tumor cells start to disseminate to BM during the initial steps of tumor development [121]. In breast cancer patients, detection of recirculated cancer cells that disseminated in BM predicts recurrence of the cancer [122]. Cancer cells can fuse with BMSCs and change their phenotype [123], or release exosomes to change the phenotype of BMSCs to cancer promoting [124]. Indeed breast tumor cells fuse spontaneously with bone marrow mesenchymal stem cells [125]. This fusion may facilitate the exchange of cellular material from the cancer cell to the BMSC rendering the fused cell more oncogenic [126]. Further, others have found the same result of this fusion and exchange of cellular material, which has been found to increase metastasis. For example, Feng et al127,found that human hepatocellular carcinoma cells with a low metastatic potential exhibit a significantly increased metastatic potential following fusion with BMSCs in vitro and in xenograft studies. In the end, the BMSCs and their molecules/exosomes, having been conditioned by tumor cells, were found to increase the probability of cancer in human patients [128]. The various phenotypes of BMSCs, including the cancerous phenotypes are difficult to distinguish [36]. In contrast, even ADSCs derived from cancer patients have been found to be safe for therapeutic development [66].
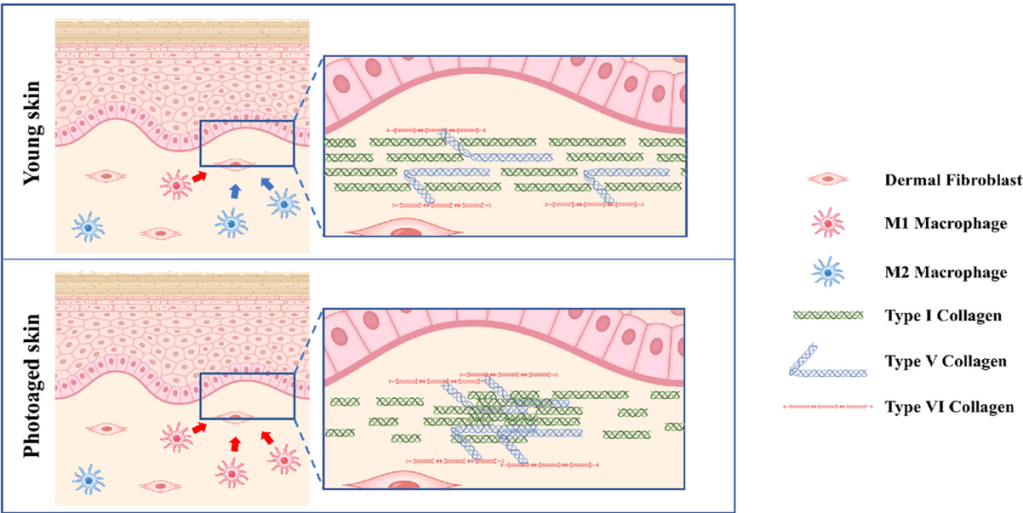
One of many reasons why ADSCs are preferred compared to BMSCs is that ADSCs express a low level of major histocompatibility complex (MHC) class I molecules and do not express MHC class II and costimulatory molecules. Even the exosomes of BMSCs express MHC class II proteins [129]. These problems in BMSCs are amplified when using donor, allogeneic BMSCs that have been replicated many times, essentially aging the cells, during expansion to develop the therapeutic. This is in contradistinction to ADSCs. Critically, when comparing experimental data of BMSCs to ADSCs from the same human donor, “ADSCs have a “younger” phenotype,” according to stem cell scientists [130]. Indeed, Burrow et al found that BMSCs have, among other negative attributes compared to ADSCs, an increased level of senescence compared to matched ADSCs. Senescent cells develop the senescence-associated secretory phenotype (SASP), a pro-inflammatory set of molecules where the local tissue effects of a SASP or specific SASP components have been found to be involved in a wide variety of age-related pathologies in vivo such as hyperplastic diseases, including cancer [131]. Whereas the use of BMSC transplants has a history of medical adverse events, including the induction of cancer in the recipient (Maguire, 2019), fat grafting, along with its constituent ADSCs, have a long history of safety in medical procedures dating back to 1893 when the German surgeon Gustav Neuber transplanted adipose tissue from the arm to the orbit of the eye in an autologous procedure to fill the depressed space resulting from a postinfectious scar [132]. Fat grafting’s long history of being safe, regardless of the harvesting techniques used in patients [120,133], has been recently reviewed by physician-scientists at Baylor College of Medicine [134]. Furthermore, physician-scientists at Stanford University School of Medicine have recently reviewed the safety and efficacy of using ADSCs to augment the outcomes of autologous fat transfers [135]. 136,have found that ADSCs and fat grafting for treating breast cancer-related lymphedema is safe and efficacious during a one year follow-on, where patient-reported outcomes improved significantly with time. In a randomized, comparator-controlled, single-blind, parallel-group, multicenter study in which patients with diabetic foot ulcers were recruited consecutively from four centers, ADSCs in a hydrogel was compared to hydrogel control. Complete wound closure was achieved for 73% in the treatment group and 47% in the control group at week 8. Complete wound closure was achieved for 82% in the treatment group and 53% in the control group at week 12. The Kaplan–Meier (a non-parametric statistic used for small samples or for data without a normal distribution) median times to complete closure were 28.5 and 63.0 days for the treatment group and the control group, respectively [137]. Treatment of patients undergoing radiotherapy with adult ADSCs from lipoaspirate were followed for 31 months and patients with “otherwise untreatable patients exhibiting initial irreversible functional damage” were found to have systematic improvement or remission of symptoms in all of those evaluated [138]. In animal models with a full thickness skin wound, administration of ADSCs, either intravenously, intramuscularly, or topically, accelerates wound healing, with more rapid reepithelialization and increased granulation tissue formation [139], and topically applied the ADSCs improved skin wound healing by reducing inflammation through the induction of macrophage polarization from a pro-inflammatory (M1) to a pro-repair (M2) phenotype [140]. I’ve discussed some of the other mechanism by which ADSCs reduce inflammation in the skin in a recent blog.
Summary
Adipose mesenchymal stem cells (ADSCs), unlike stem cells from tissues other than the skin (BMSCs and UMSCs) and stem cells from non-adult sources in the womb (UMSCs), evolved to work in the skin of adults to inhibit inflammation and to reset the innate and adaptive immune systems of the skin to a anti-inflammatory, pro-regenerative healing state to maintain and regenerate normal, non-fibrotic skin structure and function.