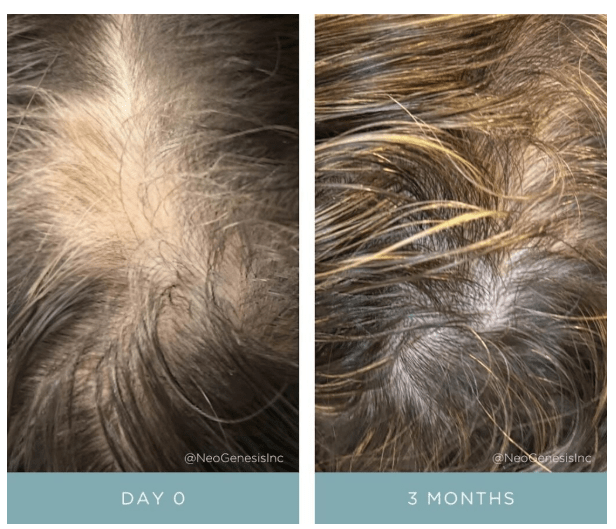
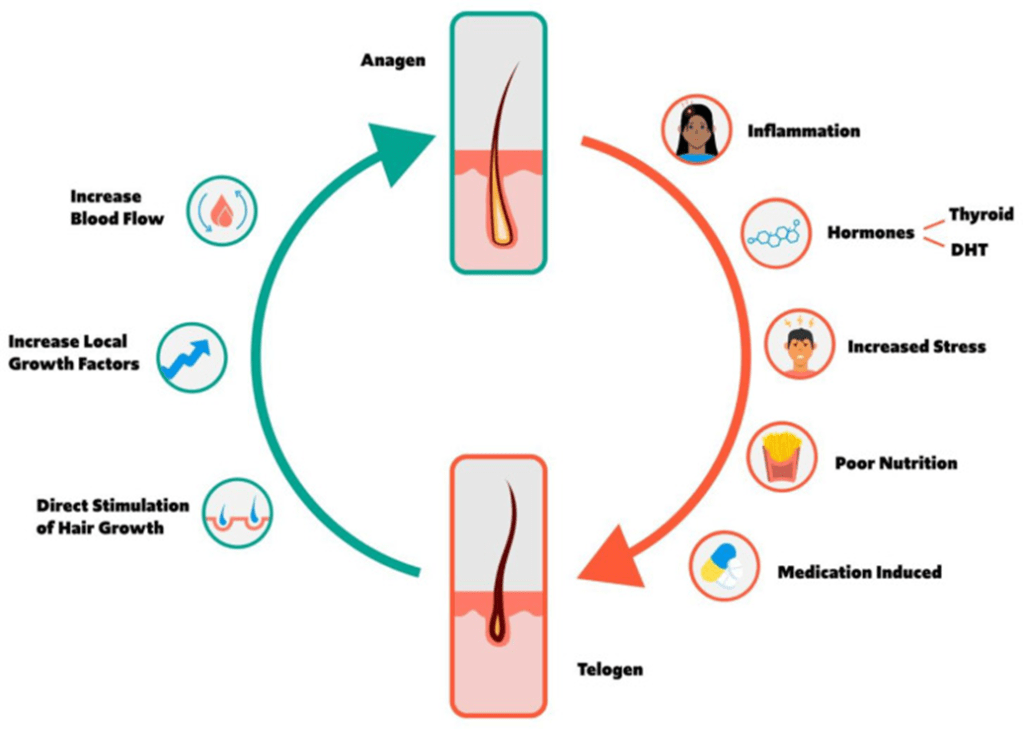
Many endogenous and exogenous factors are known to cause enlarged pilosebaceous pores. Such factors include sex, ageing, diet, chronic ultraviolet light exposure, comedogenic xenobiotics, acne, genetic and epigenetic predisposition, and seborrhoea. Most of these causative factors of enlarged pores, being exogenous and controlled by enironmental factors, means you can do something about it. There are procedures and topical products you can use to reduce pore size.
From: Yousef et al (2024)
Although the pathogenesis of enlarged facial pores is still not fully understood, three factors are thought to be key to the pathology: 1) high sebum production, 2) decreased skin elasticity around pores, and 3) increased hair follicle volume. Other factors, including chronic recurrent acne, diet, sex hormones, and skin care regimens, such as inappropriate use of cosmetics, modern Western diets, washing habits, and sun exposure, also affect pore enlargement. Many of these factors will affect the epigenetics of the skin and therefore the skin’s health and potentially pore size. Epigenetics are regulated by your environment, so there is much you can do to reduce enlarged pores.
Causes of Large Pore Size
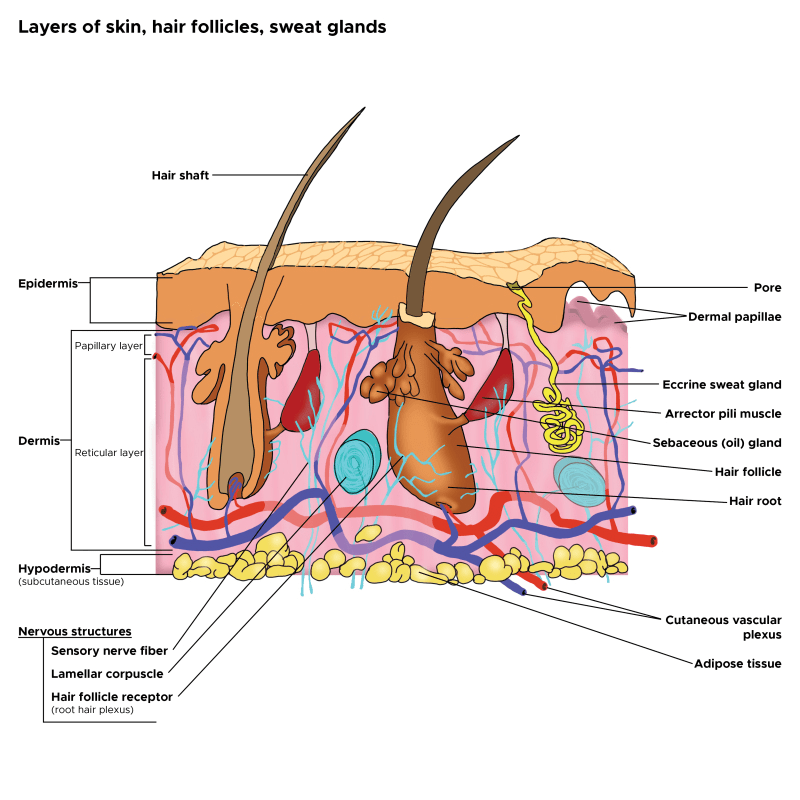
In cross-sectioned images of conspicuous, enlarged pores, a strongly undulated epidermal–dermal junction was commonly observed around a pore’s opening. Areas with this feature correlated well to the areas with larger hollows and an uneven skin tone. (Sugata et al, 2007).
Recent clinical studies have confirmed the cause of facial pore size to be multifactorial. A positive correlation of pore size and number with sebum output level has been confirmed by several studies (Roh et al, 2006; Kim et al, 2013). Enlarged pores increase with age, up to 40 years, and then stabilize or only slightly increase (Jung et al, 2018). Another significant correlation was detected between skin elasticity and pore number in two independent studies suggesting that the structure of dermis could be involved in pore widening (Kim et al, 2013; Hameed et al, 2019). Other observations found pore counts were related to wrinkle severity; and the loss of Microfibril-associated glycoprotein-1 in the hair follicle/pore area with aging and photo-exposure, indicating a lack of matrix support in the dermis (Zheng et al, 2013; Jung et al, 2018).
Both epidermal and dermal structual impairments have been identified as a cause of large pores. Microscopic imaging of pores revealed inner structural changes affecting skin, including a lower density of collagen in the deeper dermis, a thicker stroma and coarser collagen fibers forming a tubular structure around the follicle, and an irregular basement membrane ultrastructure, all of which may result in an altered distribution of skin tensions (Sugata et al, 2008; Sugiyama-Nakagiri et al, 2008; Mizukoshi and Takahashi, 2014). These ultrastructural alterations may result from inflammation, and recent data suggest inflammaging, mediated by complement activation (immune system proteins), as one of the possible inflammatory agents in the formation of enlarged facial pores (Qiu et al, 2024). Bacteria, such as Staphlacoccus aureus, infect hair follicles and pores, and the question remains, does the inflammation with this sort of infection enlarge the pore. Defects in epidermal morphology around pores have also been discovered, such as epidermis thickening and acanthosis (thickening of the stratum spinosum layer), likely indicating abnormal and possibly excessive keratinocyte proliferation ( Mizukoshi and Takahashi, 2014).
Procedures to Reuce Pore Size
Procedures, such as Micro-focused ultrasound with visualization (MFU-V), have been found to reduce pore size. MFU-V uses focused ultrasound energy to lift and tighten the skin by delivering heat to specific tissue layers beneath the skin’s surface, stimulating collagen production and causing skin tightening according to The Journal of Clinical and Aesthetic Dermatology. The visualization aspect of the procedure allows practitioners to see the underlying tissue during treatment, ensuring precise targeting and optimal results.
Topical S2RM to Reduce Pore Size – It’s Not Just the Exosome, It’s the Secretome
But are procedures needed to reduce pore size? No, the right choice of topical skin care products can significantly reduce pore size too. The secretome from adipose mesenchymal stem cells, something used in the NeoGenesis S2RM technology, significantly reduces pore size. That inflammation inducing the ultrastructual changes causing pores to enlarge can be reduced- reduce the inflammation with ADSC secretome found in the NeoGenesis S2RM technology. Remember, It’s Not Just the Exosome, It’s the Secretome that is optimal for reducing inflammation and regenerating tissue – including the tissue that constructs the pore. Changes of TEWL found that ADSC secretome can faciltate the recovery of the skin barrier function (Zhou et al, 2013), which can be explained by ADSC secretome normalizing the proliferation and migration of human primary keratinocytes as reported by Moon et al (2012). Both the epidermis (Ren et al, 2024) and dermis (Silveira et al, 2022) and hypodermis (An et al, 2021) are regenerated by ADSC secretome, with ADSC secretome containing collagen type IV needed to build the basment membrane, thereby regulating that “undulated epidermal–dermal junction” found to underly increased pore size.
I want to emphasie that inflammaging, inflammation that occurs as we age, is exposome induced. Those who eat well and live in an healthy envionment don’t suffer from inflammaging (Franck et al, 2025). As Franck et al write, “Inflammaging, as measured in this manner in these cohorts, thus appears to be largely a byproduct of industrialized lifestyles, with major variation across environments and populations.” In other words, if you live a healthy lifestyle, chronic inflammation, including inflammaging, is something you won’t suffer. This will reflect in your skin health, and your skin’s pore size.
Summary
Pore size in the skin depends on your envionment, your so-called exposome. Healthy skin is beautiful skin, including beautiful, healthy pores. Eat well to keep the skin healthy with sebum production levels normal and therefore reducing a risk factor for increased pore size. And the right choice of topical skin care products can help keep the skin healthy and pore size normal.