There are many topical vitamin C products on the market, but I needed to formulate something new because the other products are suboptimal for a number of reasons that I discuss here. Some Vitamin C products feature too much Vitamin C (15-20%) that inhibits elastin, and include alcohol at high levels to disrupt the skin barrier (penetration enhncement), resulting in the induction of inflammation in as little as 3 days of use. Alcohol-induced penetration across the skin is the result, at least in part, of stratum corneum intercellular lipid removal – in other words, alcohol destroys the fats in the skin’s barrier. Liposomal ascorbic acid is one reason the NeoGenesis vitamin C product, Vibrant C Serum, is better, and our built-in antioxidant cascade system is another. Unlike alcohol-based products with high levels of ascorbic acid (20% product and a 15% product; notice too that both of these products contain phenoxyethanol that kills cells), NeoGenesis’s Vitamin C product doesn’t destroy the skin’s barrier and doesn’t inhibit elastin, nor does it kill epithelial cells like some other Vitamin C products..
Primates, including humans, cannot synthesize vitamin C. Most other mammals (except guinea pigs), including our cats and dogs, can produce vitamin C, but primates have lost this ability due to a beneficial genetic mutation. Primates evolved to eat mostly, if not exclusively, plants and therefore obtained all the vitamin C they needed through their diets. Look at those big, strong Gorillas, they eat only plants. We primates evolved to eat plants, loaded with C, all that we needed for optimal health, and so we eventually lost the genetics to make vitamin C. In other words, we mutated, and that pesky DNA sequence for making vitamin C was a waste of energy and therefore to be efficient, evolution of primates dropped the unneeded sequence. Pretty cool how mother nature is so efficient and evolution is such as great designer, doing so without a designer. Thinking teleogically, what she said was, “you eat so much vitamin C, you don’t need to make it anymore.”
Reasons Why the Skin May Not Have Adequate Vitamin C Levels
But that was then, and this is now. People don’t eat so well these days and are stressed-out, both of which can lead to suboptimal levels of vitamin C in the skin. Even if you eat sufficent levels of Vitamin C, the transporters of vitamin C from the blood vessels don’t work well under conditions of chronic inflammation – therefore those with chronic inflammation in the skin may not be receiving adequate levels of dietary vitaimin C required for both dermal and epidermal function. The blood vessels bringing the vitamin C to the skin are no longer transporting it out of the blood into the skin – the process has been decommissoned by inflammation in the skin.
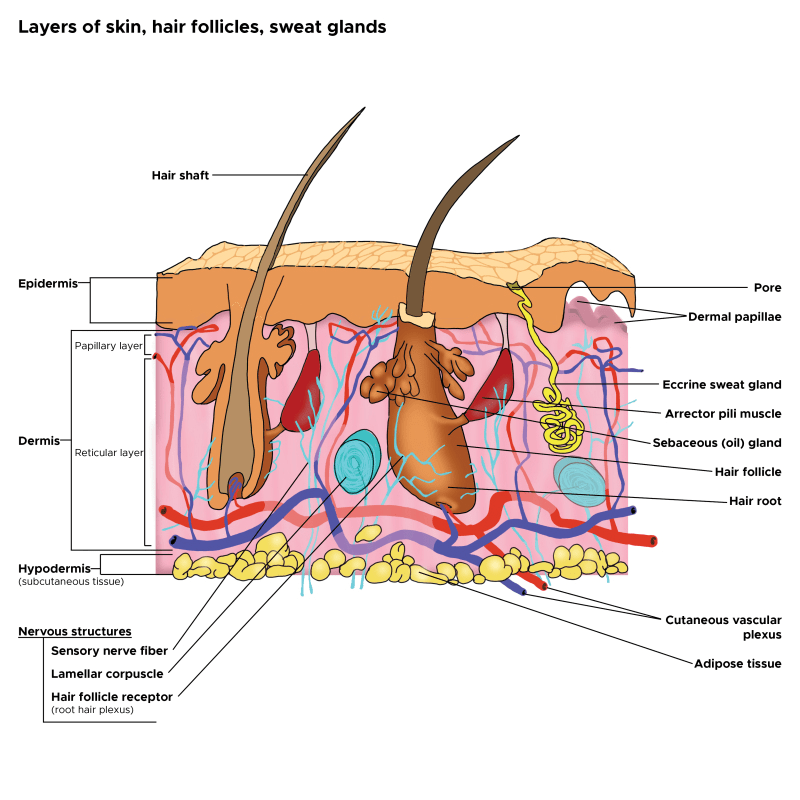
Ascorbic Acid (Vitamin C) Doesn’t Easily Penetrate the Skin
Vitaimin C (VC) has a molecular weight of only 176.12 g/mol, but it is a hydrophillic small molecule and because it interacts with water and not lipids, it dosen’t penetrate the fatty stratum coreum very well. Many companies put high levels of VC in their products, but the VC just lays on the surface of the skin.
High Levels of Vitamin C on the Skin’s Surface Oxidize (DHA an Anti-inflammatory) But Still Provide No Benefit
That 15% VC product you’re using may just sit on the surface of your skin where it will oxidize. Dehydroascorbic acid (DHA) is the oxidized form of VC, and it too has anti-inflammatory benefits just as VC does. If only it would penetrate the skin and provide benefit. Oxidized VC in the DHA form has benefits and cells, such as keratinocytes, readily take up DHA to convert it back to VC, but it needs to penetrate to the cells (keratinocytes) in the skin to give benefit. If the DHA sits on the surface of the skin too long, it can further breakdown to oxalic acid that can irritate the skin.
For those who would like to know, DHA is reduced to ascorbic acid by cytosolic reductases GSH-NADPH-dependent, lipoic acid-NADH-dependent, and thioredoxin reductase. The bottom line is that both AA and DHA are important to the skin, but longer term oxidation of AA and DHA into oxalic acid is likely detrimental.
Liposomes Carry Vitamin C Into the Skin
You’ll notice lecithin as part of the ingredient list on the Vibrant C Serum. Lecithin is part of the ingredient combination involved in making the liposomes that encapsulate the ascorbic acid. The liposomes not only protect the ascorbic acid, but importantly, carry the ascorbic acid through the stratum corneum to the deeper layers of the skin. The liposomes act without disrupting the stratum corneum and epithelial barrier function. This is different from some other Vitamin C skin care products that use alcohol as a penetration enhancer. Alcohol, especially at high concentrations, disrupts the lipid structure of the stratum corneum and reduces barrier function.
For example, some products use 15% ascorbic acid, along with an alcohol called Ethoxydiglycol, a type of ethanol. They use over 15% alcohol in their formula because the ethoxydiglycol is listed before the ascorbic acid on the product’s ingredient list – this alcohol is used as a penetration enhancer and using a high level of this alcohol in the formulation can disrupt the corneum stratum and induce irritation. In other words, this product is disrupting the stratum corneum’s barrier function.
Positive Epigenetic and Cellular Effects of Vitamin C Once It’s Absorbed Into the Skin
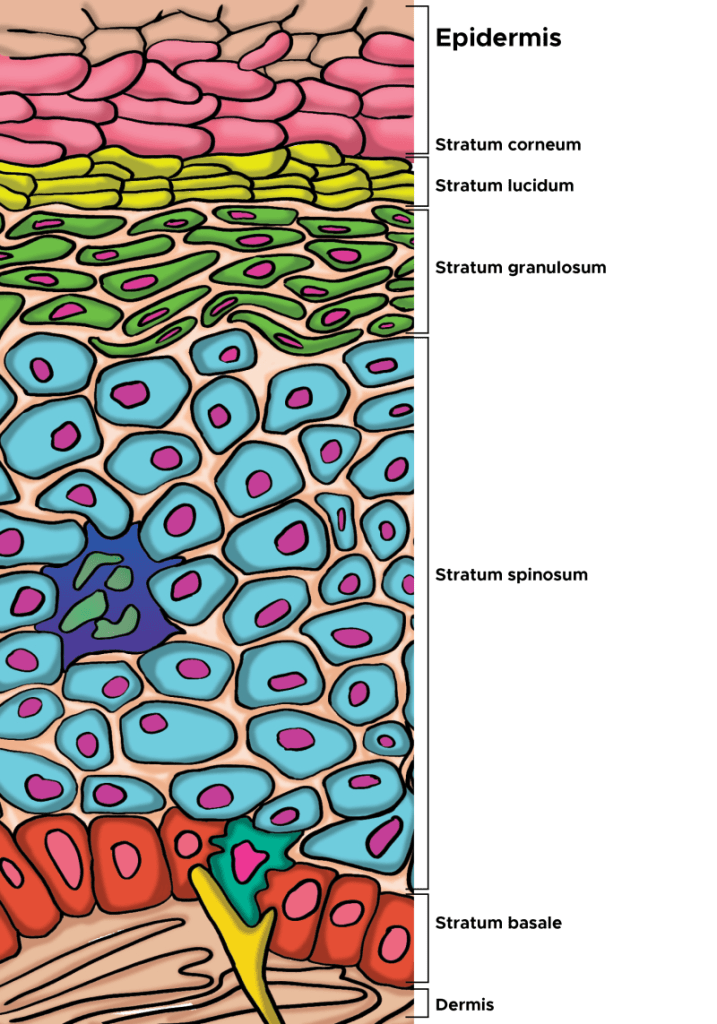
When VC penetrates to the keratinocytes, remarkable and beneficial physiological processes occur. VC increases epidermal thickness by promoting keratinocyte proliferation through the DNA demethylation of proliferation-related genes (Sato et al, 2025). This is an epigenetic effect, where VC helps to remove a methyl group that is attached to the DNA in the keratinocyte, so-called demethylation, and this allows the keratinocyte to proliferate. Part of what happens in life is that some of our DNA accumulates methyl group attachements, something that can “turn-off” the DNA. VC demethylates the DNA and turns it on again, allowing the keratinocyte to once again proliferate. It’s much more complicated than this, but for the keratinocytes this is the basic hypotheisis that scientists have brought forth.
In terms of the epidermis, L-ascorbic acid (vitamin C [VC]) is widely recognized for its antioxidant properties in the skin (Masaki, 2010), enhances collagen synthesis (Kishimoto et al, 2013), alleviates UV-induced damage to the epidermis (Kawashima et al, 2018), and inhibits melanin deposition (Sato et al, 2017). Long-term VC deficiency leads to epidermal atrophy in a mouse model with disrupted VC synthesis capabilities (Sato et al, 2012). VC promotes keratinocyte viability, induced expression of differentiation marker genes, and increased SC barrier lipids in monolayer or organotypic cultures of normal human keratinocytes (Boyce et al, 2002; Michalak et al, 2021). Together, these data suggest that VC is critical to epidermal cell proliferation and differentiation.
Collagen and Matrix Formation
Vitamin C is also crucial for the production of collagen, a protein that helps to form your skin’s overall structure and barrier and enhances your skin’s elasticity. Collagen is an important building block of the skin. In addition to stabilising the collagen molecule by hydroxylation, vitamin C also stimulates collagen mRNA production by fibroblasts in the dermis yiedling more collagen and stable collagen. Hydroxylation of collagen is a critical post-translational modification where specific amino acids, proline and lysine residues, are hydroxylated, forming hydroxyproline and hydroxylysine, respectively. This process is vital for collagen’s structural integrity, stability, and proper function, particularly within the extracellular matrix. So, Vitamin C helps to stabilize the collagen that is present, as well as to help produce new collagen.
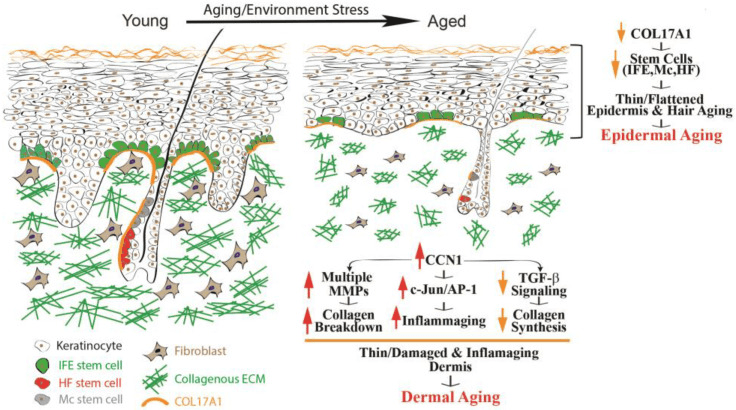
Skin collagen is a long-lived protein, having a long half-life that is estimated to be approximately 15 years, so some skin collagen can exist for much of your life. In young, healthy skin, the amount of enzymes, such as MMP (proteases) that breaks down collagen is low, and therefore, collagen degrades very slowly. However, as we age and more MMP is present, collagen ages, it starts to degrade and fragment. Unfortunately, the degraded and fragmented collagen cannot be incorporated into new collagen fibers and it accumulates within the extracellular matrix of the dermis. The presence of fragmented collagen remainders in aged dermis inhibits both fibroblast proliferation and type I procollagen synthesis by these cells, further degrading the dermis.
It’s the supporting matrix, such as collagen and elastin, that gives the skin its tightness, thickness, and firmness, but over the years, it starts to break down. That’s why skin gets saggy and thin. Again, Vitamin C is one factor, a necessary factor, to protect collagen and to produce new collagen.
Using the Optimal Amount of Ascorbic Acid: Too Much Vitamin C Inhibits Elastin
Elastin helps form the dermal matrix and helps give elasticity to the skin. Like collagen, much of the eleastin is formed in the dermis by fibroblasts. Differential effects of ascorbic acid on collagen I and elastin mRNA abundance result from the combined, marked stabilization of collagen mRNA, the lesser stability of elastin mRNA, and the significant repression of elastin gene transcription. In other words, too much Vitamin C can inhibit elastic production. At NeoGenesis, we use a topical 5% of ascorbic acid, the natural form of Vitamin C, that has been found by scientists at major university in France to rebuld the skin’s ultratructure and provide clincially visible benefits to the skin, rebuilding collagen but not destroying elastin. Moreover, our stem cell-based S2RM technology stimulates fibroblasts to form collagen and elastin, a perfect complement to our Vitamin C product..
Notice we at NeoGenesis don’t use sodium ascorbate because sodium accululates in the skin and induces inflammation, including in skin conditions such as psoraisis and autoimmune diseases. Skin sodium content has been positively and significantly correlated with classical inflammatory markers such as CRP and white blood cell count. For example, dietary salt loading can result in hypertonic sodium storage in the skin by binding to glycosaminoglycans. Topical sodium can add to the accumulation, so wherever possible, we don’t use sodium molecules in our NeoGenesis formulations.
Antioxidant Cascade – Primary and Seconday Antioxidants
You’ll find a number of primary and secondary antioxidants (they work indirectly to prevent oxidation) in the Vibrant C Serum, including: Rose Geranium (Pelargonium spp.) water, Ascorbic Acid, Magnesium Ascorbyl Phosphate, Gluconolactone, Ferulic Acid, Panthenol (a secondary antioxidant), Resveratrol, Sodium Benzoate, Hydrolyzed Rice Protein, Potassium Ascorbyl Tocopheryl Phosphate, Chinese Senna (Cassia Obtusifolia) Seed Extract, Glutathione, Curcumin (encapsulated), Honokiol, Magnolol, Ergothioneine, Soybean (Glycine Soja) Protein, and Superoxide Dismutase.
The antioxidant cascade is a series of reactions where one antioxidant molecule neutralizes a free radical and, in doing so, becomes oxidized itself. This oxidized antioxidant can then be recycled back to its active, reduced state by another antioxidant, and so on, creating a chain reaction that efficiently eliminates harmful free radicals. Antioxidants working in a cascade is where one antioxidant type regenerates another type. For example, vitamin E neutralizes lipid radicals but becomes a radical itself; vitamin C can then restore vitamin E to its active form. Enzymatic antioxidants like SOD, CAT, and GPx (Glutathione peroxidase family of enzymes) handle different reactive species at various cellular locations, creating a layered defense This process helps maintain cellular redox balance and protect against oxidative stress. Thus, different types of antioxidants work together in a cascade process by complementing, supporting, and regenerating each other’s activity, resulting in a more robust and comprehensive defense against oxidative stress than any single antioxidant could provide alone. The pathways in cellular oxidation and antioxidation are complex, involving many pathways and may different types of antioxidants. While vitamin C is important, having the other antioxidants available concurrently is critical to the cellular redox balance, the the dynamic equilibrium within a cell between oxidation and reduction reactions. Both are critical to normal cellular function.
Summary
The antioxidant cascade, involving many antioxidant types, not just Vitamin C, is important for neutralizing free radicals, but also very important in regulating cellular signaling, gene expression, and repair mechanisms. Antioxidants, such as the encapsulated curcumin found in Vibrant C Serum, can modulate key pathways (like NF-κB and MAPK) to reduce inflammation and enhance cell survival and regeneration. Delivering these many antioxidants to the cells in the skin where they can provide benefit requires good formulations where, for example, liposome and encapsulation delivery technologies are used by NeoGenesis for its Vibrant C Serum product.