Early and mid-life inflammation ia a mediator of lifelong defects in tissue maintenance and regeneration due to the inflammation aging the stem cells. Inflammation damages the extracellular matrix, DNA, and epigenetic mechanisms, all of which contribute to aging and age-related diseases.
A schematic of stem cell inflammaging (from Bogeska et al, 2022)
Inflammaging, defined as an age-related increase in the levels of pro-inflammatory markers in blood and tissues, is a strong risk factor for multiple diseases that are highly prevalent, and frequent causes of disabilities in elderly individuals but are pathophysiologically uncorrelated, i.e., everything from cancer, to skin diseases, to heart disease, and neurodegeneration. And remember, as I’ve discussed in previous blogs, inflammation in the skin can can lead to systemic inflammation.
Inflammation can wreak havoc on the body, including the skin, through a number of key mechanisms. Let’s have a look at how inflammation can damage tissue, such as by degrading the extracellular matrix, and can damage cells at the molecular level through genetic and epigenetic mechanisms. Genetic refers to how damage occurs to the DNA, and epigenetic refers to how damage occurs “above” the DNA, such as the mechanisms that control the expression of DNA – i.e., affecting how the DNA makes RNA and proteins. Inflammation can also cause misfolding in proteins, resulting in a number of dysfunctional pathways in the body, including the control of epigenetics such as protein-based epigenetics. You read that right – proteins can be inherited and dysfunctional proteins in an adult can be inherited as dysfunctional proteins in the offspring. That’s one reason why genetics and heredity don’t mean the same thing.
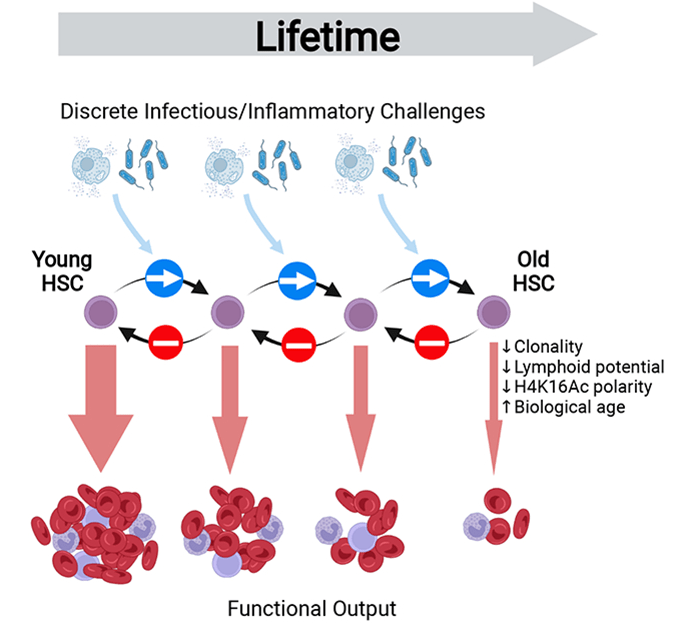
Inflammaging is a process induced by chronic inflammatory cytokine signaling that promotes accelerated damage to the extracellular matrix (ECM), stem-cell aging, and precancer stem-cell generation. Multiple different sterile and infection-associated inflammatory stimuli have been shown to provoke primitive stem cells (HSCs) to exit their long-term quiescent state and enter into active proliferation. In other words, inflammation, whether it is sterile inflammation or infection-related inflammation, drives stem cells into a state where they multiply. Therefore, chronic inflammation will induce the constant multiplication of stem cells. And every time a cell multiplies itself, mutations and consequent aging processes will occur. As I’ve said before, one of the most dangerous things a cell can do is to multiply itself.
As scientists have recently published, their work demonstrates that inflammatory stimuli can provoke a long-lasting inhibitory effect on tissue regeneration that extends far beyond the duration of the original inflammatory event, via the progressive and irreversible attrition of the functional stem cell pool. They argue that prophylactic anti-inflammatory interventions may effectively delay or prevent the evolution of age-associated pathologies, but that such treatments may hold limited capacity to rejuvenate an already aged stem cell system.
In other words, it is important to reduce inflammation even during our younger years, not just during our aged period, in order to reduce stem cell aging processes. This means eating a plant-forward diet, full of lots of fruits and vegetables, as well as using sunscreen during long sun exposures, as well as using skin products that are not inflammatory – rather using skin care products that reduce inflammation and those that help to maintain or build the skin’s barrier function.