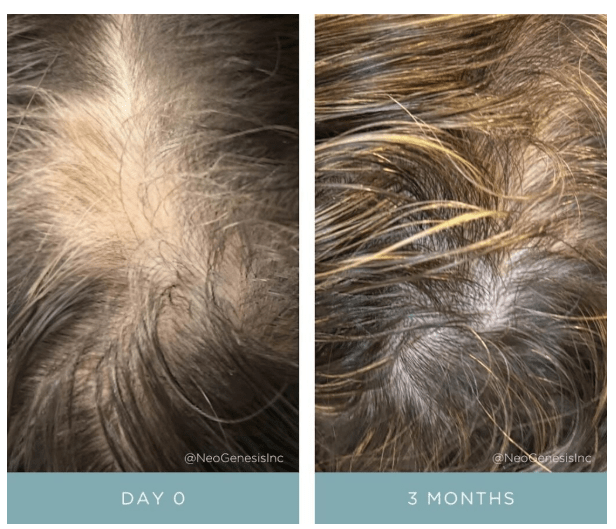
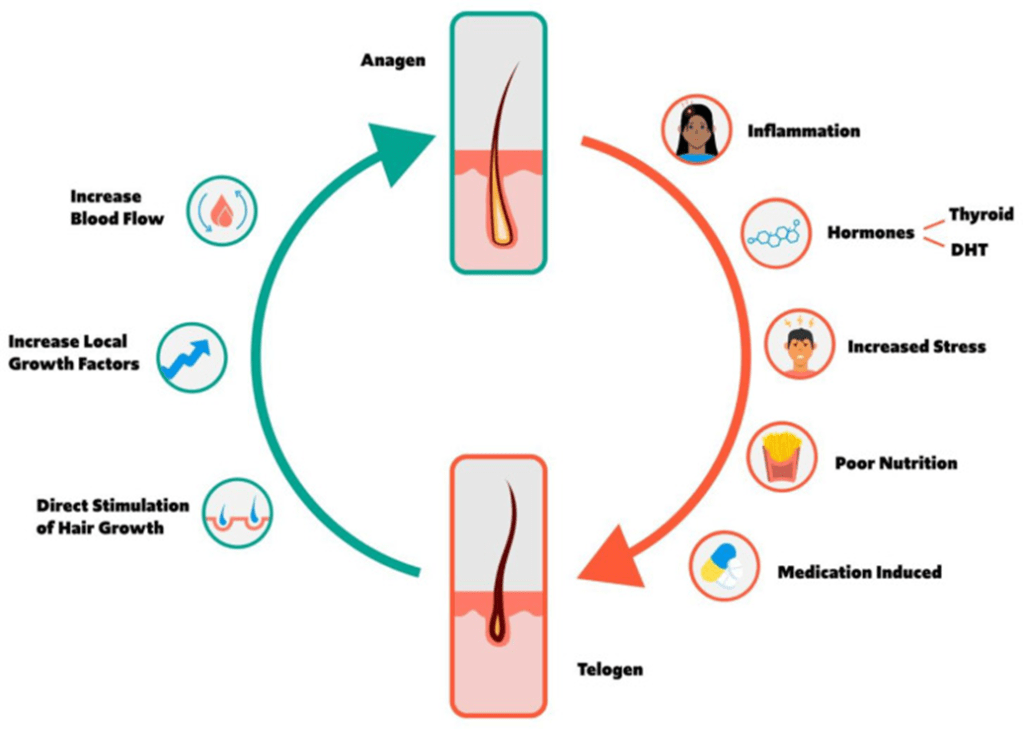
I list here some of the reasons why I formulate my skin care products using the secretome of adipose mesenchymal stem cells (ADSCs) instead of bone marrow mesenchymal stem cells or umbilical cord mesenchymal stem cells. ADSCs are better at reducing inflammation and setting the innate and adaptive immune systems into a pro-regenerative state, inducing collagen formation, and laying down that collagen in a manner that is anti-fibrotic. This is a small excerpt of my upcomming peer-reviewed publication.
Listing Efficacy of ADSCs Versus BMSCs versus UCMSCs Secretome (Exosomes + Soluble Fraction)
| Bone Marrow Mesenchymal Stem Cells (BMSCs), and the molecules they release, prolong and enhance inflammation by increasing survival and function of neutrophils (Casatella et al, 2011; Liang et al, 2024). BMSC secretome also reprograms hematopoietic stem cells to become inflammatory white blood cells (Ng et al, 2023). Under hypoxic conditions, which induces the activation of TRL4, BMSCs secrete pro-inflammatory factors and decrease the polarization of macrophages from the M1 to M2 phenotype, the M2 type being anti-inflammatory and therefore the BMSCs are promoting more inflammation (Faulknor et al, 2017; Waterman et al, 2010). Thus, BMSCs cultured in normal hypoxic conditions in the laboratory are secreting pro-inflammatory factors and when administered to wounded skin will induce inflammation by recruiting neutrophils and M1 type pro-inflammatory macrophages. |
| ADSCs have consistently exhibited much greater anti‑inflammatory capabilities, phagocytic activity, anti‑apoptotic capability activity and cell viability over BMSCs (Li et al, 2019). |
| ADSCs have been found to be highly immunomodulating cells, exceeding the suppressive effect of BMSCs by secreting more anti-inflammatory IL-6 and transforming growth factor-β1 (TGF-β1) Ceccarelli et al (2020). |
| When compared with the BMSCs- and UCSCs-treated groups, the ADSCs-treated group exhibited markedly accelerated healing efficiency, characterized by increased wound closure rates, enhanced angiogenesis, and collagen deposition at the wound site in an animal model (Cao et al, 2024). |
| ADSCs have biological advantages over BMSCs in the proliferative capacity, secreted proteins (basic fibroblast growth factor, interferon-γ, and insulin-like growth factor-1), and immunomodulatory, ant-inflammatory effects (Li et al, 2015). |
| Differences in cytokine secretion cause ADSCs to have more potent immunomodulatory effects than BMSCs (Melief et al, 2013) |
| ADSCs are better at preventing fibrosis than BMSCs (Yoshida et al, 2023). |
| Adipose mesenchymal stem cell secretome is superior to that of BMSCs because it preferentially helps to rebuild the epidermis by stimulating basal keratinocytes (Ademi et al, 2023). |
| BMSCs express much CTHRC1 protein (Turlo et al, 2023), which may help to promote fibrosis (Liu et al, 2023). |
| ADSC exosomes contain SIRT1 (Huang et al, 2020) and activate SIRT1 in other cells (Liu et al, 2021) to reduce inflammation, improve mitochondrial function, and reduce senescence. |
| ADSC exosomes reduce inflammation in endothelial cells (Heo and Kim, 2022). |
| ADSCs are considered more powerful suppressors of immune response than mesenchymal stem cells (MSCs) derived from different tissue sources, including trabecular bone, bone marrow, dental pulp, and umbilical cord (Ribeiro et al., 2013; Nancarrow-Lei et al., 2017). |
| ADSCs immunomodulatory effects exceed that of BMSCs (Melief et al., 2013). |
| ADSCs secrete higher amount of immune suppressive cytokines, such as IL-6 and transforming growth factor-β1 (TGF-β1) than do BMSCs (Soleymaninejadian et al., 2012; Melief et al., 2013; Montespan et al., 2014). |
| Bochev et al (2008) showed that ADSCs had a stronger ability to inhibit immunoglobulin (Ig) production by B cells than BMSCs. |
| Ivanova-Todorova E et al (2009) found that Adipose tissue-derived mesenchymal stem cells are more potent suppressors of the adaptive immune response through limiting dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. |
| ADSC secretome inhibits LPS-induced proinflammatory cytokines (Li et al, 2018) |
| Human ADSCs are key regulators of immune tolerance, with the capacity to suppress T cell and inflammatory responses and to induce the generation/activation of antigen-specific regulatory T cells (Gonzalez-Rey et al, 2010). |
| ADSC secretome can suppress the activation, proliferation, and function of CD8+ T cells, which are inflammatory killer T cells (Kuca-Warnawin et al, 2020). |
| ADSC secretome was able to elevate expression of M2 macrophages and modified their cytokine expression to an anti-inflammatory profile (Hu et al, 2016; Zomer et al, 2020) |
| Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodeling (Wang et al, 2017). |
| ADSC exosomes reduce inflammation and alleviate keloids by promoting mitochondrial autophagy through the PI3K/AKT/mTOR pathway (Liu et al, 2024). |
| ADSC exosomes reduce injury through the transfer of mitochondria components to neighboring cells (Xia et al, 2022). |
| ADSC secretome expedited wound healing and reduced inflammation in an animal model (Ma et al, 2021). |
| ADSC secretome promotes wound healing without leaving visible scars and was found safe when injected (An et al, 2021). |
| ADSC secretome has positive effects on granulation tissue formation and vascularization, and helps prevent fibrosis in pressure ulcers (Alexandrushkina et al, 2020). |
| Human ADSCs secrete functional neprilysin-bound exosomes that can degrade β-amyloid peptide (Aβ) that is found in the skin – cutaneous amyloidosis (Katsuda et al, 2013; Kucheryavykh et al, 2018). |
| In psoriasis and eczema the secretome from adipose mesenchymal stem cells (ADSCs), can regulate SOCS (suppressor of cytokine signaling) pathways, and modulate JAK pathways to reduce inflammation (Wang et al, 2022; Ko et al, 2023). Further, the secretome from ADSCs increases SOCS3 expression and, thus, the persistent and uninhibited expression of STAT3 by increased SOCS3 effectively ameliorates tissue injury by promoting tissue regeneration and decreasing inflammation and apoptosis (Lee et al, 2016). |
| ADSC and BMSC secretomes were characterized by the upregulation of proteins linked to ECM structure and organization and proteolytic processes compared to UCSCs, important to active involvement in tissue repair and microenvironment maintenance and suggesting their advantage for tissue-forming applications (Hodgson-Garms et al, 2025), but ADSCs are better at preventing fibrosis and reducing inflammation (Yoshida et al, 2023). |
| Fu et al (2025) found that hADSC-Exos are more effective in promoting hair follicle development compared to hUCMSC-Exos, and the secretome of ADSCs was more associated with growth processes such as nucleosome function than was the UCMSC secretome (Fu et al, 2025). |